Case Report
Austin J Nephrol Hypertens. 2014;1(2): 1009.
Stenosis at the Entrance of the Inferior Vena Cava in an 11-Years-Old Boy with Lupus Nephritis
Mengxia Li1, Yunyan Shen1, Xueming Zhu2, Qinying Xu1, Yun Zhu1, Meifeng Zeng1, QiuqinXu3, Xiaozhong Li1, Yanhong Li1,4*
1Department of nephrology, Children’s Hospital affiliated to Soochow University, China
2Department of pathology, Children’s Hospital affiliated to Soochow University, China
3Department of ultrasound, Children’s Hospital affiliated to Soochow University, Chi
4Institute of pediatric research, Children’s Hospital affiliated to Soochow University, Suzhou, China
*Corresponding author: Yanhong Li, Department of nephrology, Institute of pediatric research, Children’s Hospital affiliated to Soochow University, Suzhou, Postal code: 215003, China
Received: June 30, 2014; Accepted: July 31, 2014; Published: Aug 04, 2014
Abstract
This case report describes an 11-year-old boy with systemic lupus erythematosus (SLE) presenting with stenosis at the entrance of the inferior vena cava. The concurrent presence of the stenosis of inferior vena cava and lupus nephritis has rarely been reported in children.
Keywords: Childhood-onset systemic lupus erythematosus; Inferior vena caval stenosis; Lupus nephritis; Proteinuria; Vasculitis
Abbreviations
ANA: Antinuclear Antibody; ANCA: Anti-neutrophil Cytoplasmic Antibodies; APS: Antiphospholipid Syndrome; SLE; Systemic Lupus Erythematosus
Introduction
Childhood-onset systemic lupus erythematosus (SLE) is a multisystem autoimmune disease, having a higher frequency of major organ involvement than adults-onset SLE [1,2]. Lupus nephritis affects more than 80% of children with SLE and remains one of the most severe manifestations of SLE associated with considerable morbidity and mortality [3]. Although vascular manifestations are conditions that can occur in patients with childhood-onset SLE and are associated with lupus nephritis [2], stenosis of the inferior vena cava has rarely been reported in children with SLE and lupus nephritis.
Case Presentation
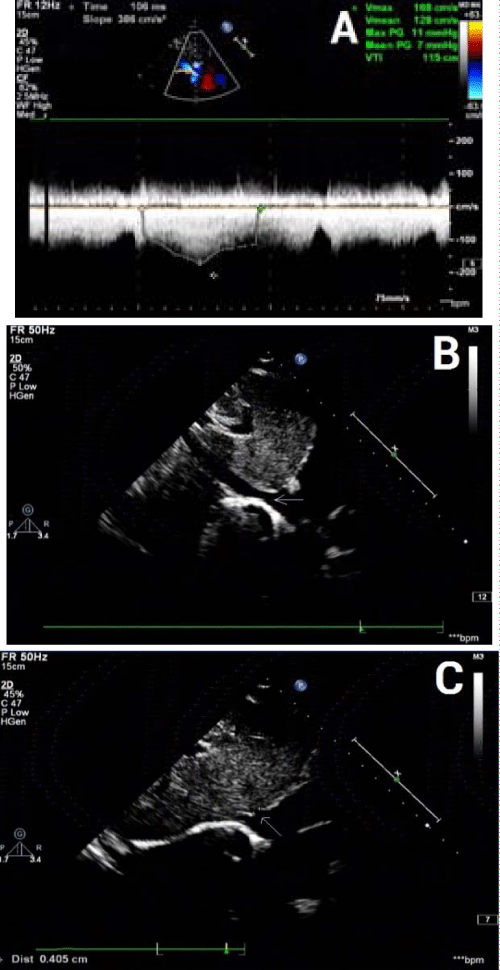
An 11 years old boy was admitted with a history of periorbital and facial edema and swelling of the ankles for three days. There was no history of gross hematuria, fever, facial rash, joint pains, or drug intake. On admission, physical examination was remarkable for periorbital and facial edema. His weight was 28 kg within normal ranges. Temperature was normal. Blood pressure was 140/90 mm Hg. There were no signs of oral ulcer, ecchymosis, petechiae, erythema, lymphadenopathy or joint swelling. The liver and spleen were not palpable below the costal margin. The laboratory results on admission were as follows: normal hemoglobin, 111 g/L; normal total white blood cell count, 5,680/mm3; lymphocyte count, 3,510/mm3; normal platelet count, 290,000/mm3; high erythrocyte sedimentation rate, 66 mm/h; normal C-reactive protein, 0.36 mg/L; normal prothrombin, partial thromboplastin, and thrombin time; and high D-dimer, 3788.0 ug /L. Urinalysis revealed: pH, 5.0; protein, +3; high red blood cell count, 17.7/ul; high white blood cell count, 26.3/ul; high epithelial cell count, 46.5/ul; no casts. The 24h urine total protein was 2181.2 mg. The serum biochemistry analysis showed: normal sodium, 138.8 mmol/L; normal potassium, 4.2 mmol/L; normal chloride, 111 mmol/L; normal blood urea nitrogen, 4.47 mmol/L; normal creatinine, 35.9 μmol/L; low total protein, 42.7 g/L; low albumin, 19.8 g/L; high total cholesterol, 9.49 mmol/L; high triglycerides, 5.77 mmol/L. Venous pH was 7.46; normal HCO3, 19.0 mmol/L; normal base excess, -3.2 mmol/L. Serum complement levels were low, with a C3 of 0.39 (normal 0.79-1.52) g/L and a C4 of 0.01 (normal 0.16–0.38) g/L. Serum immunoglobulin G (IgG), IgA, and IgM were 5.34 (normal 6.36-10.04), 1.52 (normal 0.63-1.79), and 0.86 (normal 0.29-1.41) g/L, respectively. The direct Coomb’s test was positive. The antinuclear antibody (ANA) titer was 1:320; negative test for double-stranded DNA antibody, proliferating cell nuclear antigen antibody, anti-smith antibody, anti-RNP antibody, and anti-cardiolipin antibody. Anti-neutrophil cytoplasmic antibodies (ANCA) test showed positive perinuclear ANCA (pANCA) and negative for cytoplasmic ANCA (cANCA), myeloperoxidase ANCA (MPO-ANCA), and proteinase 3 ANCA (PR3-ANCA). Blood cultures were negative for bacteria. Epstein - Barr virus DNA was negative. The CD4/CD8 ratio was 0.5 (normal 1.0-1.9). Chest X-ray showed increase in interstitial lung markings, especially on left side. The left diaphragm and costophrenic angle were obscured. An abdominal ultrasound displayed small peritoneal effusion. The stenosis of the entrances of inferior vena cava and hepatic vein was observed on the echocardiogram. The inner diameter of inferior vena cava was 11 mm, the entrance of inferior vena cava into the right atrium was 4.5 mm in diameter, and the maximal blood flow velocity of the inferior vena cava was 1.68 m/s (normal <1.0 m/s). The blood flow velocity was >1.2 m/s, indicating that the entrance was narrow on the echocardiogram. The diameter of hepatic portal vein was 3.8 mm, the entrance of hepatic vein into the inferior vena cava was 4.1 mm, and the mean blood flow velocity was 1.09 m/s (normal <1.0 m/s) (Figure 1).
Figure 1: Echocardiogram reveals the stenosis of the entrances of inferior vena cava and hepatic portal vein. A: The maximal blood flow velocity in inferior vena cava is 1.68 m/s, and the mean flow velocity is 1.28 m/s. B Arrow indicates the entrance of inferior vena cava into the right atrium, which is 4.5 mm in inner diameter. C: Arrow indicates the entrance of hepatic portal vein into inferior vena cava, which is 4.1 mm in inner diameter.
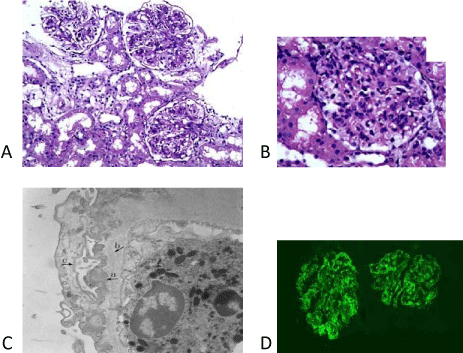
A percutaneous needle renal biopsy was performed under ultrasound guidance. On light microscopy, 13 glomeruli were identified. All of them were large and cellular, with proliferation of endocapillary and mesangial cells. The majority of glomeruli presented with neutrophilic infiltration and diminution or apparent loss of capillary lumens, as shown in Figure 2. The tubules, interstitium, and vessels were normal. Immunofluorescence showed granular global glomerular capillary wall and mesangial staining in a subepithelial distribution for C1q (+++) and C3 (++). No staining was observed with IgG, IgA, IgM, and fibrinogen. Under electron microscopy, diffuse effacement of foot process was evident, and samples presented with hump-like subepithelial electron-dense deposits.
Figure 2: Light microscopy reveals large glomeruli with proliferation of endocapillary and mesangial cells (160-250 cells/ glomerulus). A: Haematoxylin-eosin staining (magnification 200x). B: Periodic acid-Schiff staining (magnification 400x). C: Electron microscopy reveals a glomerulus with subepithelial electron-dense deposits (magnification 6000x). Arrows indicate hump-like electron-dense material deposition (a); thickening basement membrane (b); and epithelial cell foot process effacement (c). D: Immunofluorescence reveals strong staining of C1q (magnification 200x).
At this time, the diagnosis of SLE and lupus nephritis was made, based on clinical and laboratory findings. He was treated with furosemide to reduce edema and nifedipine to control blood pressure. Low molecule weight dextran and urokinase were used to improve microcirculation and prevent thrombosis. Cefodizime was used to treat infection. For the treatment of lupus nephritis, intravenous methylprednisolone therapy (250 mg/day) was given for 3 days, along with monthly intravenous cyclophosphamide (0.5 g/month). This was followed by oral prednisone (2 mg/Kg/d). His condition improved, complete remission of the nephrotic syndrome occurred after three courses of methylprednisolone plus cyclophosphamide therapy. However, the degree of stenosis of the inferior vena cava did not change at the 3-month follow-up clinic visit (Figure 1B).
Discussion
Childhood-onset SLE is a chronic autoimmune disease characterized by multisystem involvement and heterogeneity of clinical manifestations [1]. Based on the following four of the criteria, including nephrotic-range proteinuria, hypocomplementemia, positive ANA, and positive direct Coombs test in absence of hemolytic anemia, our patient was classify as having SLE according to the American College of Rheumatology classification criteria for SLE [4].
This patient initially presented with edema, nephrotic-range proteinuria, hypoproteinemia, hyperlipidemia, mild hematuria, and transient hypertension. Renal biopsy revealed endocapillary proliferative glomerulonephritis and positive immunofluorescence for C1q and C3. Since the case is unusual in that renal biopsy did not exhibit typical pathological findings of lupus nephritis, a wide range of conditions had to be considered in the differential diagnosis for nephrotic syndrome. In addition, the existence of stenosis of the inferior vena cava, which is not a typical vascular manifestation associated with SLE, may also complicate the diagnosis.
The presence of stenosis of the inferior vena cava and seropositive ANCA in the patient might be suspicious of ANCA-associated vasculitis, which often affects the kidneys [5]. However, ANCA-associated glomerulonephritis is characterized by little or no staining for immunoglobulin or complement in glomeruli on immunofluorescence microscopy [6]; and this disease was not fit for our patient. The positive of ANCA could be a helpful diagnostic marker for SLE [7].
In addition, our patient had a hypercoagulable state, indicating that antiphospholipid syndrome (APS) had to be considered. APS, as the most common acquired hypercoagulation state of autoimmune disorder in children, is also associated with SLE and mainly affects vessels. However, it has been suggested that APS are frequently associated with multiple positive antiphospholipid antibodies in children [8]. The negative anti-cardiolipin antibody in our patient did not support the diagnosis of APS.
Although previous studies suggest that inflammation and/or thrombosis may affect almost any vessel in patients with SLE [2], there was no evidence to support that the stenosis of the inferior vena cava was due to inflammation or thrombosis within the vessel, based on our patient’s clinical and laboratory findings. The concurrent presence of stenosis of the inferior vena cava without evidence of lupus vasculitis made it difficult to determine whether venous stenosis was induced by SLE. The relationship of stenosis of the inferior vena cava with SLE and lupus nephritis in our case is uncertain.
Renal biopsy revealed positive immunofluorescence for C1q and C3, but negative for IgG, IgA, and IgM, in this case. Although the presence of a “full house” pattern of glomerular immunoglobulin and complement deposits on immunofluorescence is not always necessary for diagnosis of SLE in children, both C1q nephropathy and C3 glomerulopathy were considered. C1q nephropathy manifests dominant or co-dominant immunofluorescence staining for C1q in a predominantly mesangial distribution. Although it bears some resemblance to lupus nephritis, C1q nephropathy has very rarely been described in patients with positive antinuclear antibody serologies and abnormal serum complements [9]. C3 glomerulopathy is a new classification, and the glomerular pathology is characterized by the isolated deposition of C3 in the glomerulus [10]. So, this kind of disease could be excluded.
Conclusion
The presence of hypocomplementemia, positive ANA, positive Coombs test, and seropositive ANCA, as well as positive immunofluorescence for C1q and C3 in glomeruli on immunofluorescence microscopy, suggests that nephrotic syndrome was caused by lupus nephritis itself. Because concurrent inferior vena cava stenosis and lupus nephritis have rarely been reported in children, whether venous stenosis is a risk factor for clinical outcome in patients with SLE is not established. Long-term follow up to confirm the diagnosis of SLE and exclude ANCA-associated glomerulonephritis is necessary in the future.
References
- Barsalou J, Levy DM, Silverman ED. An update on childhood-onset systemic lupus erythematosus. See comment in PubMed Commons below Curr Opin Rheumatol. 2013; 25: 616-622.
- Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. See comment in PubMed Commons below Pediatr Clin North Am. 2012; 59: 345-364.
- Punaro MG. The treatment of systemic lupus proliferative nephritis. See comment in PubMed Commons below Pediatr Nephrol. 2013; 28: 2069-2078.
- PPons-Estel GJ, Wojdyla D, McGwin G Jr, Magder LS, Petri MA, Pons-Estel BA, et al. The American College of Rheumatology and the Systemic Lupus International Collaborating Clinics Classification criteria for systemic lupus erythematosus in two multiethnic cohorts: a commentary. Lupus 2014; 23: 3-9.
- Lee T, Gasim A, Derebail VK, Chung Y, McGregor JG, Lionaki S, et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. See comment in PubMed Commons below Clin J Am Soc Nephrol. 2014; 9: 905-913.
- Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. See comment in PubMed Commons below J Am Soc Nephrol. 2010; 21: 1628-1636.
- Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. See comment in PubMed Commons below Blood. 2012; 120: 3007-3018.
- Avcin T. Antiphospholipid syndrome in children. See comment in PubMed Commons below Curr Opin Rheumatol. 2008; 20: 595-600.
- Markowitz GS, Schwimmer JA, Stokes MB, Nasr S, Seigle RL, Valeri AM, et al. C1q nephropathy: a variant of focal segmental glomerulosclerosis. See comment in PubMed Commons below Kidney Int. 2003; 64: 1232-1240.
- Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC. C3 glomerulopathy: a new classification. See comment in PubMed Commons below Nat Rev Nephrol. 2010; 6: 494-499.