Review Article
Austin J Nephrol Hypertens. 2014;1(2): 1010.
Hemodialysis: Diffusion and Ultrafiltration
Ramin Sam*
Department of Medicine, University of California, USA
*Corresponding author: Ramin Sam, Department of Medicine, San Francisco General Hospital, University of California, 1001 Potrero Ave, SFGH 100, San Francisco CA 94143, USA
Received: July 17, 2014; Accepted: July 30, 2014; Published: Aug 01, 2014
Introduction
Normal kidneys perform at least two major functions; first they remove a variety of toxins and second they remove excess fluid from the body. In addition, the kidneys are important metabolic organs involved in glucose metabolism and production of erythropoietin, renin, vitamin D, etc. The kidneys accomplish its toxin-excreting function by first filtering large amounts of plasma to form a filtrate with a composition resembling that of the plasma except for the absence of proteins and protein-bound substances. Much of the filtered fluid is reabsorbed leaving behind a volume of urine that contains the waste products that require removal from the body every day. All of this is a continuous process that occurs twenty four hours a day. The goals of hemodialysis are mainly also two fold. First hemodialysis removes kidney failure-related toxins and second it is capable of removing excess water and salt. Hemodialysis accomplishes these goals in a manner different from what a normal kidney does. Plasma is passed outside of the body into a dialyzer (i.e., a filter) containing a large number of hollow fibers. These fibers separate the plasma from the dialysate and provide a large surface area for diffusion to take place. The dialysate is formed by mixing purified water with proper amounts of electrolytes and other essential constituents (such as glucose). As opposed to our own kidneys, there is some barrier to movement of molecules, even for those of relatively small sizes such as vitamin B12 (molecular weight 1,355). Also there is no reabsorption with dialysis, making adding needed small molecule to the dialysate, the only way of not removing these molecules from the body.
Hemodialysis as currently practiced is not a continuous process, unlike our kidneys. Even though the removal, during the time of dialysis, of small molecules such as urea is not dissimilar to the removal provided by the normal kidney, the overall clearance of urea is only about one tenth of that of the normal kidneys. This is because people commonly only receive dialysis for 12 hours or less a week whereas the normal kidney labors every second of the day. The dialysate composition is now standardized in most dialysis units with room allowed for small variations. However theoretically there is unlimited possibility to vary the dialysate composition based on the needs of the patient. During hemodialysis treatments, water and sodium are not ordinarily removed by diffusion but rather through the process of ultrafiltration. Ultrafiltration is commonly accomplished by lowering the hydrostatic pressure of the dialysate compartment of a dialyzer, thus allowing water containing electrolytes and other permeable substances to move from the plasma to the dialysate. The sodium level of an ultrafiltrate is not too distant from that of plasma. Finally, noteworthy is the fact that the dialyzability of a substance depends not only on the size of the substance but also on the permeability of the dialyzer membrane and the degree of protein-binding of that substance.
Contact between Blood and Dialysate
In order for hemodialysis to take place blood and dialysate have to meet inside the dialyzer even though the two fluids are separated by a semi-permeable dialyzer membrane (Figure 1). Dialyzers come in different sizes but are often cylindrical and about 20-30 cm (8-12 inches) long. The main job of the dialysis machine (Figure 2) is to make the blood and dialysate go through the dialyzer.
Figure 1: Dialyzer.
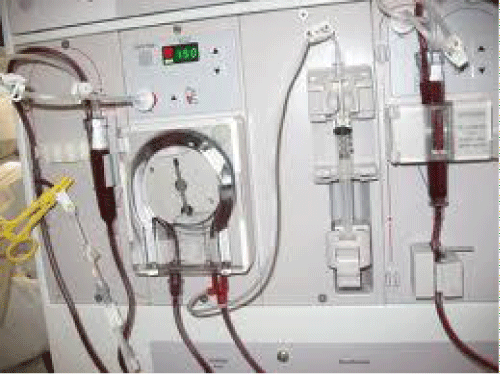
Figure 2: Dialysis machine.
After the blood leaves the patient through her or his vascular access which can be in the form of a catheter or an arteriovenous access, it first goes through a drip chamber which helps to get rid of air in the tubing. The next step is the blood pump which pumps the blood ideally at a rate of 300-500mL/min, followed by the dialyzer. After the blood leaves the dialyzer, it enters another drip chamber and finally an air detector. The latter can detect large air bubble and stop the flow of blood if those bubbles are present. A dialysis machine strives to make sure that there is no air in the blood lines as dialysis patients are usually on dialysis for 3-4 hours at a time with no one continuously watching them. If there are lots of air bubbles, they can cause a severe air embolism. Both pre- and post-dialyzer are ports where medications such as erythropoietin, heparin, blood products, or fluids can be given (Figure 3).
Figure 3: Dialysis process.
Formation of dialysate: purification of water
The goals of purifying water for dialysis are three fold: 1. To remove ions such as calcium which are in abundance in tap water; 2. To remove chlorine and chloramines as they are added to city water to kill off bacteria; 3. To reduce the bacteria and endotoxin levels to an acceptable range. Before this can be done, the water for dialysis has to come in at a high pressure. Usually the water is first passed through a water softener which removes the calcium and the magnesium present. Then the water moves to a charcoal filter whose main job is to remove the chloramine which can lead to hemolysis if present in high amounts [1]. Most commonly then, a reverse osmosis filter is used to remove more than 95% of the remaining ions and some bacteria. Alternatively or sometimes in addition, a deionizer is used to remove the remaining ions. In this case however, bacteria are not removed [2], requiring an ultrafilter or ultraviolet radiation to destroy bacteria that are present. Ultrapure water refers to even more strict limits on bacteria counts and bacterial toxin levels which are accomplished by specialized filters, not employed routinely at most United States dialysis centers.
Formation of dialysate: The acid and base concentrates
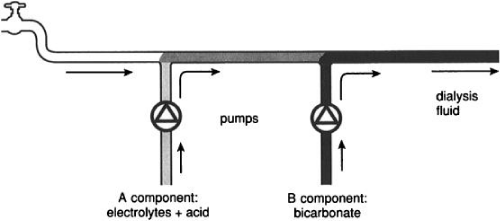
By using the “three stream method” (Figure 4) the water that has been purified is then mixed in appropriate proportions with an acid concentrate and a bicarbonate concentrate of a dual concentrate dialysis system to constitute the final dialysate before being pumped into the dialyzer. The acid and the bicarbonate concentrates have to be separated until the final mixing right before the dialyzer as the calcium and magnesium in the acid concentrate will precipitate out when in contact with the high bicarbonate level in the bicarbonate concentrate. However, upon mixing the acid concentrate, the bicarbonate concentrate and the purified water within the dialysis machine to make the final dialysate, the small amount of acid in the acid concentrate will titrates an equal amount of bicarbonate in the bicarbonate concentrate to produce carbon dioxide and water (i.e., carbonic acid). This latter acid will lower the pH of the final dialysate to the realm of 7, a pH that will allow the divalent cations to remain in solution. There are several proportioning systems to do the above mixing. The most popular system is the 45X system which mixes 1 part of the acid concentrate, 1.72 parts of the bicarbonate concentrate and 42.28 parts of the purified water. Thus if the final dialysate potassium concentration is targeted to be 2mEq/L, and it does not contain potassium beforehand then 45 times as much potassium or a level of 90mEq/L has to be added to the acid bath. Knowing the exact proportions of concentrates and purified water used in a proportioning system is required if one wishes to add a compound to either the acid or the bicarbonate concentrate of a dual concentrate dialysis system for transfer by dialysis into the blood. The dialysate flow rate into the dialyzer is usually between 500-800mL/min in conventional, thrice-weekly hemodialysis sessions.
Figure 4: Three stream method of preparing a bicarbonate-based hemodialysate using a dual-concentrate, proportioning machine. Figure obtained from: Ledebo I with permission for reproduction.
Dialysate sodium
After water, sodium is the most abundant molecule in the plasma. In fact out of every 295 particles in the plasma (serum osmolality), 137 are sodium and another 137 are the corresponding negatively charged particles such as chloride and bicarbonate. As such sodium is the main determinant of extracellular fluid volume. Consequently, salt restriction is paramount in the treatment of oliguric or anuric dialysis patients with fluid excess. Since sodium restriction is often not strictly observed, most dialysis patients are over hydrated (often exhibiting edema).Dialysate sodium concentration is often set in the realm of 135-140mEq/L. A higher dialysate sodium level such as that used in sodium profiling has been employed by some centers to abstract tissue and intracellular fluids into the intravascular space so that blood volume and blood pressure could be better maintained during dialysis. However, this approach is not often favored on account of the development of interdialytic hypertension and also it leads to thirst and large intradialytic weight gain. Excess sodium and water are ordinarily removed during dialysis sessions via the process of ultrafiltration.
Dialysate potassium
Potassium excretion drops and often seizes with advanced renal failure and in order to maintain proper balance, this ion has to be removed with hemodialysis. The standard dialysate potassium level in most dialysis units is 2mEq/L. With the use of such a level, the serum potassium value at the end of a dialysis session is often fairly low, in the low 3s or the high 2s. This is because, during dialysis, the removed potassium comes mainly from the relatively small extracellular compartment, thus causing the immediate post-dialysis serum level to be low even though the amount removed is not that substantial. In this regard, the average amount removed by a dialysis treatment is in the realm of 50mEq [3]. However after a few hours the serum potassium concentration would bounce back to a value not too far from the predialysis level as a result of entry of intracellular potassium into the blood. Since the serum potassium level can be quite low for a short period of time during dialysis, the heart might develop arrhythmias as a result. In fact arrhythmias occurring during dialysis are often caused by the temporary hypokalemia above mentioned. This problem can be addressed by increasing dialysate potassium concentration. This hypokalemia-induced arrhythmogenic effect is potentiated by the concomitant administration of digitalis-related preparations.
Dialysate chloride
Chloride constitutes the main anion in the plasma and dialysate. Dialysate chloride level is purposely fashioned higher than that in plasma in order to comply with the Gibbs-Donnan effect [4].
Dialysate glucose
In order to make sure there is no risk for hypoglycemia, especially in the case of diabetic patients, the standard dialysis bath contains 200 mg/dL (10.1mmol/L) of dextrose (i.e., glucose monohydrate, MW 198) which will translate to 182 mg/dL (10.1mmol/L) of glucose (MW 180). If the patient is really hyperglycemic (serum glucose concentration of > 200 mg/dL [11 mmol/L]), then some glucose will actually be removed by dialysis. More recently, since normal serum glucose level is in the neighborhood of 100 mg/dL (5.6mmol/L) and a higher than normal serum glucose level may have undesirable effects, some centers have opted to use a dextrose level of 100 mg/dL in the dialysate.
Dialysate calcium
The calcium bound to albumin is not well dialyzable. Thus it is only the ionized calcium present in the serum that matters with respect to dialysis. Normal ionized calcium concentration in the serum is usually in the order of 4.8-6 mg/dL (1.2 to 1.5mmol/L). However dialysis patients are often a little hypocalcemic. Standard calcium baths nowadays contain 2.5mEq/L (1.25mmol/L) of calcium. The calcium bath can be adjusted to 3mEq/L, and sometimes to 3.5mEq/L or 2mEq/L or less if needed in particular patients.
Dialysate magnesium
Magnesium is similar to potassium and usually the levels increase with renal failure. In dialysis patients the goal would be to remove magnesium with dialysis and usually magnesium concentration in the dialysate is in the order of 1.2 mg/dL (0.5 mmol/L or 1 mEq/L). We are unaware of any studies on adding magnesium to the dialysate in the unusual dialysis patient with hypomagnesemia.
Dialysate acetate
The acid concentrate (pH 3 or thereabouts) usually contains 180 mmol/L of acetic acid if a 45X acid concentrate system is used. This amount of acetic acid can provide 180/45 = 4 mmol/L of acetic acid in the final dialysate. In the latter, this 4mmol/L of hydrogen from the 4mmol/L of acetic acid will titrate 4 mmo/L of bicarbonate to form carbon dioxide and water as stated above. As the result of this process, although the final dialysate has lost 4 mmol/L of bicarbonate due to the titration, it has gained an equivalent amount of acetate, namely, 4mmol/L. Thus, the sum of bicarbonate and acetate concentrations in the final dialysate will be equal to the original bicarbonate concentration if acetic acid had not been added. Acetate, after having been dialyzed into the blood, will be metabolized into bicarbonate under ordinary circumstances (in the absence of hypotension, etc).
Vitamin B12 and vitamin C
Vitamin B12 is normally present in the serum at a concentration of about 250-900 pg/mL. At a molecular weight of 1,355, vitamin B12 is not easily dialyzable with low-flux dialysis. However it is possible to remove a significant amount of vitamin B12 with high-flux dialyzers under certain conditions. A recent study looking at water soluble vitamin levels in patients with extended hours hemodialysis did not find an increased incidence of vitamin B12 deficiency but found some cases of vitamin C (which is much smaller at a molecular weight of 176 g/mole) deficiency [5]. The authors concluded that it is important for dialysis patients to take renal vitamins (which are mainly B complex vitamins) while on dialysis. The water soluble vitamins are not routinely added to the dialysate.
Vancomycin
Certain medications that we give to patients are dialyzable. Vancomycin (MW1,486), poorly dialyzable when using low-flux dialyzers, can be dialyzed using high-flux dialyzers. The peak therapeutic serum vancomycin levels are in the order of 10-15 ng/mL. One can remove fairly large amounts of vancomycin with long high-flux dialysis treatments along with substantial ultrafiltration. In one study, the authors were able to lower the serum vancomycin level of one patient from 99ng/mL to 36ng/mL with three long hemodiafiltration treatments using high-flux dialyzers [6]. Vancomycin is not routinely added to the dialysate.
The composition of the base bath: Sodium and bicarbonate
The base concentrate contains either sodium bicarbonate alone or a mixture of sodium chloride and sodium bicarbonate. The final bicarbonate concentration in the dialysate is in the realm of 35-40 mEq/L. This is clearly higher than the serum bicarbonate concentration in patients with renal failure who are often acidotic. Thus bicarbonate administration with dialysis is the main method of correcting the metabolic acidosis of dialysis patients.
Other substances that have been added to the dialysate: Ethanol
Ethanol is commonly used in the treatment of ethylene glycol and methanol poisoning, at least before Fomepizole came into the market. Having a small molecular weight of 46 and not protein-bound, ethanol is removed easily by dialysis and has to be replenished in patients poisoned by the above-mentioned toxic alcohols and being treated with dialysis so that a proper alcohol level in the blood can be maintained. A convenient way of achieving a preferable blood level is to add ethanol to the dialysate. A final dialysate ethanol concentration in the neighborhood of 100 mg/dLis usually targeted [7].
Other substances that have been added to the dialysate: Iron
Iron in the form of ferric pyrophosphate complexed with sodium citrate is being investigated as iron supplementation in hemodialysis patients [8].
Other substances that have been added to the dialysate: Citric acid
Citric acid when added to an acid concentrate to provide a citrate level in the final dialysate in the realm of 0.8-1.3 mmol/L (2.4-4 mg/dL) can serve as a regional anticoagulant for the blood within a dialyzer. In addition, comparable anticoagulant effects have also been obtained when citrates are added to a bicarbonate concentrate instead [9]. The problem with citrate is that it chelates with calcium and lowers the serum ionized calcium concentration by 10-15%. As such the blood calcium concentration should be closely monitored in patients treated with citrate-enriched dialysis [7].
Other substances that have been added to the dialysate: Urea
Urea has been added to the dialysate to prevent dialysis disequilibrium syndrome in patient with very high starting serum urea nitrogen concentrations [10].
Summary of diffusion with hemodialysis
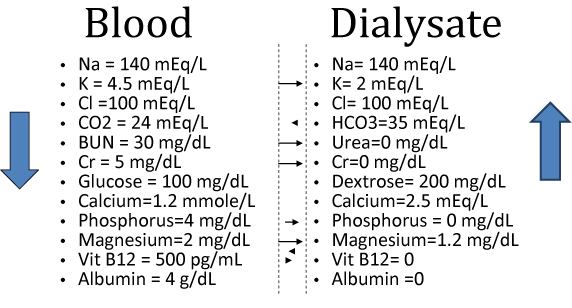
Figure 5 summarizes the diffusion of all the molecules available in the dialysate and some in the blood that are not normally present in the dialysate. The formal definition of diffusion in dialysis is movement of solutes as a result of random molecular motions across a semipermeable membrane down its concentration gradient. With dialysis most of the toxin removal is accomplished by diffusion.
Figure 5: Summary of the diffusion of common substances between the dialysate and the serum. Cr = creatinine.
Ultrafiltration
In hemodialysis water is removed by hydrostatic ultrafiltration which is a pressure phenomenon. The pressure on the dialysate side is lowered and water moves from a place of higher pressure to one of lower pressure, i.e., out of the plasma. This is how fluid gets removed every time a patient is dialyzed. The osmolality of the dialysate is actually lower than that of the plasma because of mainly the lack of urea in the dialysate.
Removal of sodium with dialysis
Sodium that is gained between dialysis treatments is ordinarily removed during dialysis by ultrafiltration not by diffusion. As serum sodium concentration often does not change much between dialysis days while patients gain weight during the interdialytic period, they must have retained sodium and water in the same ratio as that present in plasma. The ultrafiltrate removed during dialysis and the corresponding plasma contains closely similar amounts of sodium and water.
Isolated ultrafiltration
In isolated ultrafiltration, no dialysate is used and no diffusion is involved. The pressure in the dialysate compartment is lowered in order to allow movement of fluid out of the blood space. With isolated ultrafiltration small molecular weight substances are also removed as the semipermeable membrane is still permeable to the same molecules as before. However they are removed at the same rate as water and the serum concentration of a permeant small molecule such as urea does not change.
Hemofiltration
In hemofiltration large amount of fluid (i.e. 2-3 liters/hour) is removed through ultrafiltration from the patient. To maintain a normal blood volume, an equal volume of a replacement fluid needs to be infused back to the patient. The replacement fluid resembles plasma and contains sodium, chloride, calcium, dextrose and bicarbonate. Other molecules such as potassium may also be present. With hemofiltration the urea (and other permeant small molecules) concentration in the blood will drop as there is no urea in the replacement fluid and urea is removed with the fluid removal. When hemofiltration is performed in continuous slow fashion, it is called continuous veno-venous hemofiltration or (CVVH), as opposed to continuous veno-venous hemodialysis (CVVHD).
Hemodiafiltration
Hemodiafiltration refers to renal replacement therapy where dialysis and hemofiltration are performed simultaneously using the same dialyzer/filter. This is the most efficient way of doing renal replacement therapy. In the continuous form it is called continuous veno-venous hemodiafiltration or (CVVHDF).
Rebound
Rebound refers to the fact that some substances such as urea or potassium which are in large part intracellular have low concentrations in the blood as a result of renal replacement therapy but the levels rise sharply in the next few hours. This phenomenon happens because the still high postdialysis intracellular level allows diffusion of the substance into the extracellular space. The degree of rebound depends on the rate of dialysis.
Access recirculation
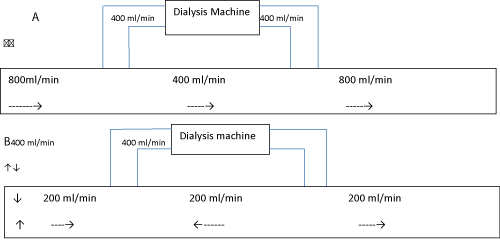
Access recirculation only has to do with the vascular access and mainly results from decreased access flow which in turn is usually the result of either a narrowing in the outflow vein or in the incoming artery. The low access flow will cause some of the just dialyzed blood to go in a loop and be dialyzed again (Figure 6).
Figure 6: Panel A shows a patient with no access recirculation with blood flowing in the access always from left to right (The flow through the access is 800 ml/min with blood flow through the dialysis machine being 400 ml/min, that leaves 400 ml/min of blood continuing to go through the access. After the blood from dialysis comes back to patient the blood flow through the access becomes 800 ml/min again), while panel B shows a patient in whom 50% of the blood that comes into the dialyzer is recirculated blood that was just dialyzed (Because of a stenosis of the access somewhere in the arm, the blood flow through the access is only 200 ml/min, the dialysis machines blood flow is still set at 400 ml/min. The only way the following could happen is for some of the blood that was just dialyzed to flow backward in the arm and go be dialyzed again).
Clearance with dialysis
Dialyzer clearance is defined as the volume of plasma or blood from which a given substance has been removed completely in a given time period. The dialyzer clearance of a substance depends on four factors: 1. Surface area of the dialyzer. 2. Blood flow rate. 3. Dialysate flow rate. 4. Permeability of that substance with respect to the dialyzer membrane. It is important to point out that dialyzer clearance is not dependent on the serum concentration of the substance. High efficiency dialyzers have large surface area for dialysis, whereas high flux dialyzers have larger holes to permit passage of bigger molecules. The trend in recent years has been to use a dialyzer which is both high efficiency and high flux.
Case example pertaining to dialysis
The following three cases are in order to better understand clearance concept with dialysis which has lots of similarities with normal kidneys also.
Case 1
Patient #1 and #2 are the same weight, total body water, diet and have same creatinine generation. They both have normal renal function and bilateral hypernephroma. On day zero, both patients undergo bilateral total nephrectomy. Patient #1 starts 3 times a week dialysis on day 0. Patient #2 starts 3 times a week dialysis on day 15, when the serum creatinine is 16. Dialysis prescription is same in both patients. What is the serum creatinine on patient #1 and patient #2 three months after nephrectomy and why?
- Answer: The serum creatinine would be the same.
- Clearance = D X V/P Where D is the Dialysate concentration of creatinine, V is the dialysate flow rate and P is the plasma concentration of creatinine.
- Clearance of patient #1 = clearance of patient #2 (same dialysis prescription).
- D X V is the creatinine excretion which is equal to creatinine production.
- Creatinine production of patient #1 = creatinine production of patient #2.
- Thus plasma creatinine of patient #1 has to equal the plasma creatinine of patient #2.
Case 2
Patient #1 and patient #2 have the same urea generation, total body water, weight and diet. Patient #1 has been getting dialyzed 8 hours 6 days a week for the last year. Patient #2 has been getting dialyzed 4 hours 3 times a week for the last year. Other than the time, the rest of the dialysis prescription is the same in both patients. All the dialysate from both patients is collected over one week. Which patient would have higher urea excretion over the week?
- Answer: Urea excretion would be the same.
- In steady state, urea generation is equal to urea excretion.
- Both patients are generating the same amount of urea, thus urea excretion must be the same.
- Patient #1 would have a much lower average serum urea than patient #2.
- It is important to point out that we don’t care about how much waste products we remove but care about how much is left behind.
Case 3
Patient #1 and patient #2 have the same urea generation, total body water, weight, and diet. They both have a serum BUN of 100 mg/ dL on day 0. Patient #1 starts dialysis 4 hours 3 times a week on day 0. Patient #2 starts dialysis 2 hours 6 times a week on day 0. What would be the serum BUN on patient #1 and patient #2 on day 6?
- Answer: The serum BUN on patient #2 would be a little lower.
- First hour of dialysis serum BUN maybe 100 and concentration gradient of BUN between blood and dialysate is high.
- Second hour of dialysis serum BUN maybe 75 and concentration gradient is still high.
- Third hour of dialysis serum BUN maybe 40.
- Fourth hour of dialysis serum BUN maybe 25 and little urea is being removed.
- Thus most efficient dialysis occurs at the beginning of dialysis.
- However the main reason patient #2 will have a higher clearance is because of rebound. With the first patient the 3rd and 4th hour of dialysis happen right after the first 2 hours and there is no chance for rebound to have occurred, while in the second patient, the 3rd and 4th hour of dialysis happens the next day after rebound has taken place.
Adsorption
Some large molecules even though they are too big to go through the dialyzer membrane, still get removed a little bit because of adsorption. Adsorption refers to the molecule sticking to the dialyzer membrane and being removed without actually going across the membrane. For example some dialyzer membranes remove ß2 microglobulin by adsorption [11].
URR versus KT/V
URR is the urea reduction ratio and represents the percentage decrease in serum urea concentration with dialysis. KT/V (also known as single-pool Kt/V) represents the number of times the entire body’s urea distribution volume (close to the volume of total body water) gets cleared of urea in the dialysis treatment. K represents dialyzer clearance, T duration of dialysis and V urea distribution volume. A KT/V of one indicates the entire body’s urea distribution volume has been cleared of urea during that particular dialysis treatment. KT/V is a better measure of dialysis adequacy as it takes into account the clearance related to ultrafiltration and urea generation during the dialysis treatment [11].
Single pool KT/V versus eKT/V
The eKT/V differs from its single pool counterpart by taking into account rebound. A few hours after dialysis the serum urea concentration rises rapidly. This rise is not taken into account by single pool KT/V; however eKT/V (equilibrated Kt/V) takes that into account. Even though eKT/V is a better measure of dialysis adequacy, it is often underused in dialysis centers. This may be because of the fact that the guidelines for KT/V to be at least 1.3 to 1.4 were validated by the single pool KT/V and not by eKT/V. The eKT/V generally is about 0.2 lower than the single pool KT/V; however an eKt/V value depends on the rate of dialysis. With higher rates of dialysis, the difference between single-pool Kt/V and eKt/V is exaggerated.
Hypertension with dialysis
Hypertension is very common in dialysis patients. It is classically assumed that hypertension in dialysis patients is volume- mediated. Thus the treatment for this hypertension first involves removing more fluid with dialysis. Such removal is however not possible in many cases because of several problems. Most importantly many patients drop their blood pressures to very low levels with fluid removal thus limiting the amount of fluid that can be removed. The reason that many patients cannot tolerate excess fluid removal well is because the removal rate has exceeded the refilling rate (the rate of entry of extracellular fluid into the capillaries) of the capillaries of the body. Having patients take their blood pressure medicines after dialysis maybe helpful at times. More frequent dialysis or longer treatments may also be help to remove more fluid but often nursing staff shortage gets in the way. High sodium baths, sodium modeling with the dialysates, treatment with midodrine and carnitine infusions have been tried with varying success. Salt restriction is always an attractive option but is rarely followed through. Apart from intradialytic hypotension, some patients are unable to tolerate more fluid removal because of the development of severe cramps with dialysis. Again the options for treating this problem are limited. Patients with intradialytic hypotension are often treated with intravenous saline administration, thus contributing to the fluid excess. Lastly some patients just refuse to remove more fluid as they perceive themselves to be thin already and fear any more weight loss. This is despite the fact the dialysis only removes the salt and the water and does not cause real non-excess-fluid weight loss. In spite of various obstacles, salt restriction and more frequent and longer dialysis treatments are the best solutions to the problem of intradialytic hypotension.
Anemia with dialysis
Anemia in dialysis patients is mainly because of a lack of erythropoietin. Erythropoietin administration has made a tremendous improvement in the lives of dialysis patients. Recent studies however have shown that lower hemoglobin targets (hemoglobin of not higher than 12 g/dL) may actually be better than having targets closer to normal hemoglobin levels. Not uncommonly dialysis patients are still anemic despite adequate erythropoietin administration. This is most commonly because of relative iron deficiency. Administration of intravenous iron during dialysis is a common practice. However, the long term effects and toxicity of intravenous iron administration have really not been studied adequately so far.
Metabolic bone disease with dialysis
Dialysis patient usually have low calcium, high phosphorus and high parathyroid hormone levels in the serum. Lowering the phosphorus levels is commonly difficult despite apparently adequate dialysis treatments and large doses of phosphorus binders. Dietary compliance should also be routinely advocated. If more frequent and longer dialysis treatments become routine the problems with phosphorus control often improve. The high parathyroid hormone levels are often treated with vitamin D analogues and, if such therapy is not successful, with the addition of calcimimetics. Calcimimetics are very effective in lowering the parathyroid hormone levels to the point that the need for parathyroidectomy has decreased since their introduction. However many patients are unable to tolerate the doses of the calcimimetics needed to suppress parathyroid hormone to desired levels mainly because of gastrointestinal side effects.
Malnutrition with dialysis
Malnutrition remains common with dialysis patients and inadequate dialysis is one of the frequent causes. Like malnutrition with cancer and other severe chronic diseases, malnutrition in dialysis patients remains very hard to treat. It is important for the nephrologist to look for edema and treat hypertension with fluid removal because if the patient is losing weight and the dry weight is not adjusted then fluid overload becomes a problem.
Conclusion
Normal kidney function allows us to remove very intricately the toxins and fluid that we do not need in a slow continuous way, while retaining needed substances that are required for our wellbeing. This is performed by first filtering large amounts of plasma indiscriminately except for very large molecules and then reabsorbing what is needed in a very complex and efficient way. At times however the system fails to function perfectly as for example in lithium poisoning(a large amount of lithium is reabsorbed by the kidneys despite the presence of toxic serum lithium levels and the clearance of lithium would actually be higher with dialysis than with normal kidneys). However this situation is fairly uncommon. In contrast the dialyzer membrane is much less permeable even to middle-sized molecules. With dialysis there is also no reabsorption. Thus any molecule that is large enough to be filtered and needed for wellbeing has to be added to the dialysate, in order for it to be retained in the body. Currently the only compounds that we add to dialysate routinely are sodium, chloride, potassium, calcium, bicarbonate, magnesium, acetate, and dextrose. It is possible in the future the list of these substances will expand especially if we continue to use higher flux dialyzers. With hemodialysis one supplements calcium and bicarbonate, while removing potassium, magnesium, and urea and other toxins using diffusion. Water and sodium are removed by ultrafiltration.
References
- Sam R, Haghighat L, Kjellstrand CM, Ing TS. Hemolysis during hemodialysis, In: Handbook of dialysis therapy. Nissenson AR, Fine RN, editors. 4th edn, Saunders, Philadelphia, PA. 2008. 457-466.
- Ward RA, Ing TS. Product water and hemodialysis solution preparation, In: Handbook of dialysis. Daugirdas JT, Blake PG, Ing TS, editors. 4th edn. Lippincott, Williams and Wilkins, Philadelphia, PA. 2007. 79-86.
- Hou S, McElroy PA, Nootens J, Beach M. Safety and efficacy of low-potassium dialysate. See comment in PubMed Commons below Am J Kidney Dis. 1989; 13: 137-143.
- Petitclerc T. Estimation of mass transfer through a hemodialyzer: theoretical approach and clinical applications. See comment in PubMed Commons below Artif Organs. 1998; 22: 601-607.
- Coveney N, Polkinghorne KR, Linehan L, Corradini A, Kerr PG. Water-soluble vitamin levels in extended hours hemodialysis. See comment in PubMed Commons below Hemodial Int. 2010.
- Gatchalian RA, Popli A, Ejaz AA, Leehey DJ, Kjellstrand CM, Ing TS. Management of hypophosphatemia induced by high-flux hemodiafiltration for the treatment of vancomycin toxicity: intravenous phosphorus therapy versus use of a phosphorus-enriched dialysate. Am J Kidney Dis. 2000; 36: 1262-1266.
- Sam R, Vaseemuddin M, Leong WH, Rogers BE, Kjellstrand CM, Ing TS, et al. Composition and clinical use of hemodialysates. See comment in PubMed Commons below Hemodial Int. 2006; 10: 15-28.
- Gupta A, Amin NB, Besarab A, Vogel SE, Divine GW, Yee J, et al. Dialysate iron therapy: infusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. See comment in PubMed Commons below Kidney Int. 1999; 55: 1891-1898.
- Cheng YL, Yu AW, Tsang KY, Shah DH, Kjellstrand CM, Wong SM, et al. Anticoagulation during haemodialysis using a citrate-enriched dialysate: a feasibility study. See comment in PubMed Commons below Nephrol Dial Transplant. 2011; 26: 641-646.
- Doorenbos CJ, Bosma RJ, Lamberts PJN. Use of urea containing dialysate to avoid disequilibrium syndrome, enabling intensive dialysis treatment of a diabetic patient with renal failure and severe glucophage induced lactic acidosis. Nephrol Dial Transplant. 2001; 16: 1303-1304.
- Daugirdas JT. Physiologic principles and urea kinetic modeling. In: Handbook of dialysis, Daugirdas JT, Blake PG, Ing TS, editors. 4th edn. Lippincott Williams and Wilkins, Philadelphia, PA. 2007; 25-58.