Research Article
Austin J Nephrol Hypertens. 2014;1(3): 1011.
Aliskiren Reduces Proteinuria Through Changes in Central and Peripheral Hemodynamics in Chronic Kidney Disease Patients
Hiromichi Suzuki*, Tsutomu Inoue, Tomohiro Kikuta, Tsuneo Takenaka and Hirokazu Okada
Department of Nephrology, Saitama Medical University, Japan
*Corresponding author: Hiromichi Suzuki, Department of Nephrology, Saitama Medical University, 38 Moroyama-machi, Iruma-gun, Saitama, 350-0495 Japan
Received: August 12, 2014; Accepted: August 20, 2014; Published: August 22, 2014
Abstract
Recently, a newly developed direct rennin inhibitor (DRI), aliskiren, has been shown to have a persistent depressor action throughout the day in hypertensive patients. However, only a few reports compared Reno protection by aliskiren to angiotensinogen receptor blockers (ARBs) in proteinuric chronic kidney disease (CKD) patients.
Methods: Measurements of urinary protein excretion (Upro) were carried out before and 6 months after the change from other ARBs to aliskiren. Twenty three patients who persistently had values of Up ro >1.0 g daily despite the treatment with antihypertensive drugs including ARBs were considered as candidates. Initially, aliskiren was started at 150 mg once daily in the evening, and increased up to 300 mg once daily in the evening. Home BP was utilized for determination of BP control. In addition to BP measurements, pulse wave velocity (PWV) and second SBP (SBP2), an index of central BP, were evaluated before and 6 months after aliskiren administration. Renal function was evaluated by estimated glomerular filtration rate (eGFR).
Results: Administration of aliskiren reduced Upro (2.16 ± 0.91 to 1.62 ± 1.08 g/day, P<0.05), accompanied with decreases in systolic BP, SBP2 and PWV. Moreover, reduction of Upro was positively correlated with those of changes in SBP (r=0.46, P<0.05), SBP2 (r=0.48, p<0.05) and PWV (r=0.48, P<0.05). Renal dysfunction evaluated by eGFR did not progress during the study period (before 70.46 ± 9.56, after 68.00 ± 12.24 ml/min/1.73m2).
Conclusion: The results of the present study suggest that aliskiren exerted its renoprotective action through improvement of central and peripheral hemodynamics.
Keywords: Chronic kidney disease(CKD); Pulse wave velocity(PWW); central arterial pressure, home blood pressure
Abbreviations
CKD: Chronic Kidney Disease; PWW: Pulse Wave Velocity
Introduction
Blockade of the Renin-Angiotensin-Aldosterone System (RAAS) using angiotensinogen converting enzyme inhibitors (ACEi) and angiotensinogen receptor blockers (ARB) has been advocated for the management of proteinuria in chronic kidney disease (CKD) patients [1]. Aliskiren, a direct renin inhibitor (DRI) inhibits the enzyme renin by binding to its catalytic site, thus blocking the RAAS at its point of activation. Angiotensinogen I, angiotensin II, and aldosterone levels decrease, and their hemodynamic effects are abolished. Aliskiren impedes efferent arteriolar vasoconstriction and diminishes the glomerular filtration fraction, salt and water absorption [2-4]. Although the effects of ACEi and ARBs result in an increase in plasma renin concentration and activity, the inhibition of the RAAS might be incomplete. In this regard, aliskiren might be superior to ACEis and ARBs. Recently, Lizakowski et al. [5] reviewed several small studies on the antiproteinuric effects of aliskiren and found marked reduction of proteinuria and albuminemia in diabetic and non-diabetic CKD patients. Previous reported studies were mainly carried out in the protocol of add-on style [6]. However, there are no definitive studies comparing the renoprotective effects of aliskiren with that of available ARBs. In the present study, the efficacy of aliskiren was assessed on blood pressure (BP) control and renoprotection in CKD patients in whom reductions of proteinuria were more than 1.0 g daily in spite of administration of ARBs.
Methods
This study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from all patients.,/
This study was a prospective and observational cohort study conducted in a 6 months treatment period with aliskiren.
Patients
CKD patients in stages 2 and 3 who had proteinuria >1.0 g daily despite treatment with ARBs for more than 1 year, were considered as candidates for the change from ARBs (10 patients on olmesartan, 6 patients on candesartan, 5 patients on valsartan, and 2 patients on telmisartan) to aliskiren. CKD stages 2 and 3 were defined as the estimated glomerular filtration rate (eGFR) being >30 mL/ min/1.73m2. Exclusion criteria were as follows: types 1 and 2 diabetes mellitus, hypokalemia (>5.5 mEq/L), severe cardiovascular diseases, treated with corticosteroid or immunosuppressant, past history of a major surgery within 6 months, and other diseases that physicians had elected to treat.
Office BP was not measured and patients were given instructions on how to measure and record their own BP at home. BP measurements were recorded at least twice a week at home in the sitting position - once in the morning before breakfast within 30 min of awakening, and once in the evening just before dinner. Home BP measurements were made using the HEM 401C (Omron Life Science Co. Ltd, Tokyo, Japan), a semi-automatic device that operates on the cuff-oscillometric principle and generates a digital display of systolic and diastolic BP and pulse rate [7]. Pulse wave velocity (PWV) was measured using an automatic waveform analyzer (form PWV/ ABI; Omron Colin, Co., Ltd., Komaki, Japan). All individuals were examined after resting in the supine position for at least 5 minutes and radial artery pulse waveform was recorded by an automated tonometric system HEM-9000AI (Omeron Healthcare, Kyoto, Japan) with patients in a sitting position. The HEM-9000AI algorithm automatically performed online detection of the second peak (late systolic inflection) based on the second maxima of the fourth derivative of the radial pressure wave form to determine the late or second SBP (SBP2), an index of central BP [8]. SBP2, an index of CAP, is well correlated with central arterial pressure (CAP) measured simultaneously by the direct catheter method [9,10].
BP was evaluated by the average of 20 measurements at home during a month and doses of antihypertensive agents except aliskiren were adjusted. The target levels of BP were less than 130/80 mm Hg.
All of the subjects were registered to replace their single daily morning ARBs to aliskiren when their daily proteinuria was >1.0 g regardless of the levels of BP.
The serum creatinine, 24-hr urinary excretions of creatinine and protein (Upro), and hematologic and serum tests including urea, uric acid, blood urea nitrogen (BUN), electrolytes, etc. were obtained at the beginning and end of the baseline period and during every month of follow-up.
Statistical analysis
Data are expressed as mean ± standard deviation. The differences from baseline to 6 months were tested using the Student’s test for paired data.
Correlations between various characteristics were determined using Pearson’s correlation test. All statistical tests were two-sided with significance level of 0.05, and were performed using the SAS Release 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
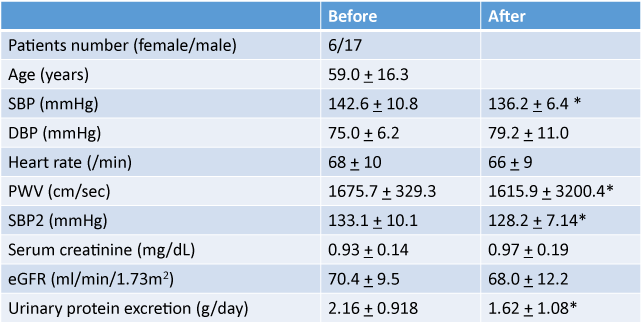
The mean age of all subjects was 59.0 ± 16.3 years and female to male ratio was 6/17. Underlying renal diseases were: Ig A nephropathy, 7; nephrosclerosis, 6; membranous nephropathy, 2; and unknown, 8. Patient baseline values are presented in Table 1. Before the start of aliskiren, average values were: SBP at home was 142.6/75 ± 10.8/0 mm Hg; PWV was 1675.7 ± 329.3 cm/sec; eGFR was 70.4 ± 9.5 ml/min/1.73 m2; and urinary protein excretion was 2.16 ± 0.91/g Cr. Administration of aliskiren resulted in significant reductions of SBP, SBP2 and PWV with a significance.
Table 1: Changes in variables before and after administration of aliskiren
Correlations
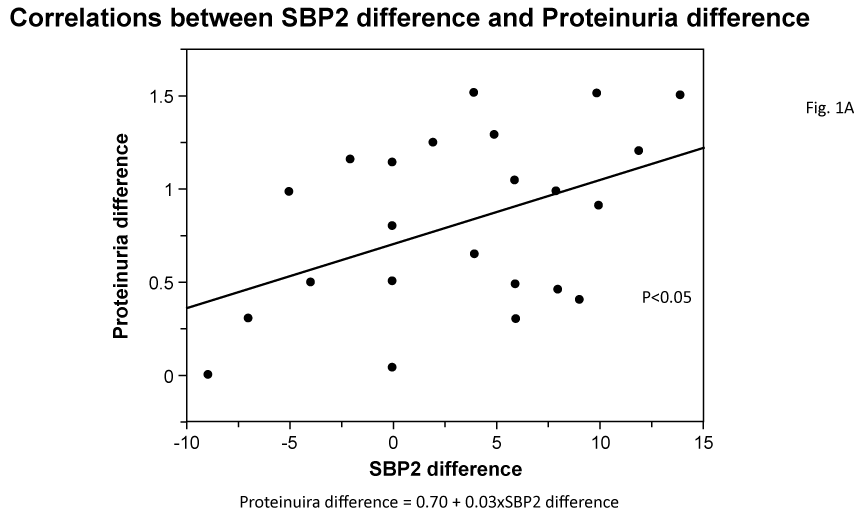
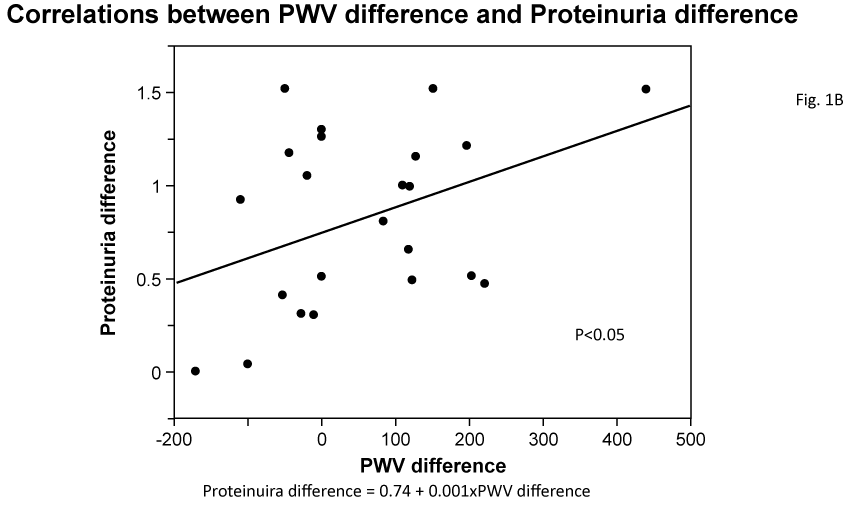
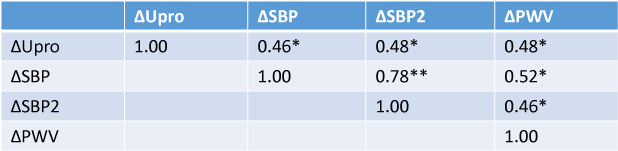
Univariate correlations among variables are shown in Table 2. Difference in Upro between before and after the administration of aliskiren significantly correlated with the before and after differences for SBP2 and PWV (Figure 1A, 1B). Also, differences in SBP, SBP2 and PWV were significantly correlated with each other. Also, differences in SBP (data not shown), SBP2 and PWV were significantly correlated with each other.
Figure 1a: Correlations between SBP2 difference and Proteinuria difference
Figure 1b: Correlations between PWV difference and Proteinuria difference
Table 2: Correlations among Variables
Discussion
In the present study, changes from ARBs to aliskiren decreased Upro despite treatment with ARBs in CKD patients. Moreover, reduction in Upro positively correlated with peripheral and central hemodynamic changes.
For the first time, aliskiren was introduced as an add-on drug to standard treatment consisting of the recommended renoprotective dose of ARBs [11]. The AVOID trial (Aliskiren Combined with Losartan in Type 2 Diabetes and Nephropathy) [12] clearly demonstrated that add-on aliskiren reduced the mean albuminuria by 20%, showing a reduction of 50% or more in 24.7% of the patients who received aliskiren compared to 12.5% in those who received a placebo. In contrast, the ALTITUDE study (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints) [13] was stopped because the study was unlikely to show a benefit from aliskiren and there was an increased incidence of non-fatal stroke, renal complications, hyperkalemia and hypotension in the patients randomized to the aliksiren group. Aliskiren in combination with and ACEi or ARB is now contraindicated in all diabetic patients, and preferably avoided in non-diabetic patients with impaired renal function [14]. Consequently, there have been few reports discussing the role of aliskiren in treatment of CKD.
In a number of trials in patients with hypertension, aliskiren monotherapy was generally as effective as hydrochlorothiazide, ramipril, lisinopril, irbesartan, atenolol, valsartan, and losartan at reducing BP in short term [15-18].
The renoprotective effects of the RAAS-inhibiting drugs have been shown to be in part independent of the reduction in systemic BP, as well as involving the normalization of glomerular hyper perfusion and hyper filtration, restoring glomerular barrier function, and reducing the non-hemodynamic effects of angiotensin II and aldosterone [1].
Constant treatment with an ACEi or ARB has been shown to return Ang II and aldosterone to their pre-treatment levels [19]. Conceivably, this could be a suboptimal suppression of RAAS activity causing incomplete reduction of urinary protein excretion. In the present study, aliskiren reduced both BP and urinary protein excretion during 6 months compared with values before the start of aliskiren. Aliskiren, in contrast to ACEi and ARBs, decreases plasma renin activity by approximately 50-80%.
Degree of reduction of Upro was well correlated with peripheral and central hemodynamics.
Recently, Tomiyama et al. [20] clearly demonstrated in middle aged healthy Japanese men that proteinuria was associated with an increased brachial-ankle PWV, radial AI, and SBP2 although only 2.9% of patients that participated in this study had proteinuria. Similarly, Weir et al. [21] demonstrated that SBP and PWV were both associated with variations in proteinuria in patients with CKD in a cross-sectional cohort of patients with CKD.
Finally, the crosstalk between peripheral and central circulation needs to be discussed. There are several important forms of crosstalk between the macro- and microcirculation in hypertension. The first is the effect of an enhanced pulse pressure on small arteries.
It is suggested that at the level of the kidney, central as well as peripheral hemodynamics contribute to producing albuminuria and other forms of renal damage [22]. Although the present study was a relatively small one, it demonstrated that the recurring patterns of proteinuria could be abolished by changes from ARBs to aliskiren.
Study Limitations
The following limitations of this study are recognized: short follow-up of 6 months, no placebo control arm, no double blind trial, and small number of subjects. A single-arm study with open-label might be an improper setting to compare the effects of ARBs on Upro and BP control. However, in this pilot study, we attempted to alert clinicians to the importance of patient selection in using aliskiren in proteinuric CKD patients. Moreover, BP reduction is also an important therapeutic target. This is supported by the evidence from large-scale trials in the treatment of patients with overt proteinuria and CKD, indicating the importance of lower SBP treatment goal [23- 25]. In the present study, reduction of BP was not very large, but the levels of BP measured at home were higher compared with the targets of office BP. Alternatively, it is conceivable that the combination of aliskiren and reduction of BP may reduce UPro in CKD patients.
The reduction of BP might be more effective by improvement of peripheral and central hemodynamics assessed by PWV and central BP.
In conclusion, the present study suggested that aliskiren exerted its renoprotective action through improvement of central and peripheral hemodynamics.
References
- Tylicki L, Lizakowski S, Rutkowski B. Renin-angiotensin-aldosterone system blockade for nephroprotection: current evidence and future directions. J Nephrol. 2012; 25: 900-910.
- Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a Novel Orally Effective Renin Inhibitor, Provides Dose-Dependent Antihypertensive Efficacy and Placebo-Like Tolerability in Hypertensive Patients. Circulation. 2005; 111: 1012-1018.
- Trimarchi H. Role of aliskiren in blood pressure control and renoprotection. Int J Nephrol Renovasc Dis. 2011; 4: 41-48.
- Juncos L. Direct renin inhibition: extricating facts from façades. Ther Adv Cardiovasc Dis. 2013; 7: 153-167.
- Lizakowski S, Tylicki L, Rutkowski B. Direct renin inhibition--a promising strategy for renal protection? Med Sci Monit. 2013; 19: 451-457.
- Persson F, Rossing P, Parving HH. Direct renin inhibition in chronic kidney disease. Br J Clin Pharmacol. 2013; 76: 580-586.
- Suzuki H, Nakamoto H, Okada H, Sugahara S, Kanno Y. Self-measured systolic blood pressure in the morning is a strong indicator of decline of renal function in hypertensive patients with non-diabetic chronic renal insufficiency. Clin Exp Hypertens. 2002; 24: 249-260.
- Suzuki H, Dogi M, Takenaka T. Various approaches for vascular health in elderly women. Clin Exp Hypertens. 2013; 35: 295-299.
- Takazawa K, Kobayashi H, Kojima I, Aizawa A, Kinoh M, Sugo Y, et al. Estimation of central aortic systolic pressure using late systolic inflection of radial artery pulse and its application to vasodilator therapy. J Hypertens. 2012; 30: 908-916.
- Takenaka T, Kikuta T, Watanabe Y, Inoue T, Takane H, Ohno Y, et al. Validation of carotid blood pressure assessment by tonometry. J Hypertens. 2012; 30: 429-432.
- Onuigbo MA. Analytical review of the evidence for renoprotection by renin-angiotensin-aldosterone system blockade in chronic kidney disease - a call for caution. Nephron Clin Pract. 2009; 113: c63-69, discussion c70.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK, AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008; 358: 2433-2446.
- Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012; 367: 2204-2213.
- Health Sciences Authority (HAS) ADRN. Available from:URL: https://ww.hsa.gov.sg. 2012.
- Andersen K, Weinberger MH, Egan B, Constance CM, Ali MA, Jin J, et al. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial. J Hypertens. 2008; 26: 589-599.
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, et al. Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation. 2009; 119: 417-425.
- Krone W, Hanefeld M, Meyer HF, Jung T, Bartlett M, Yeh CM, et al. Comparative efficacy and safety of aliskiren and irbesartan in patients with hypertension and metabolic syndrome. J Hum Hypertens. 2011; 25: 186-195.
- Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W, et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007; 20: 11-20.
- Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: is oral renin inhibition the solution? Expert Opin Pharmacother. 2007; 8: 529-535.
- Tomiyama H, Odaira M, Matsumoto C, Yamada J, Yoshida M, Shiina K, et al. Effects of Moderate-to-Severe Impairment of the Estimated Glomerular Filtration Rate and of Proteinuria on the Central Hemodynamics and Arterial Stiffness in Middle-Aged Healthy Japanese Men. Int J Nephrol. 2011; 2011: 427471.
- Weir MR, Townsend RR, Fink JC, Teal V, Anderson C, Appel L, et al. Hemodynamic correlates of proteinuria in chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6: 2403-2410.
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008; 105: 1652-1660.
- Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, et al; RENAAL Study Group. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003; 163: 1555-1565.
- Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001; 135: 73-87.
- Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, et al. Independent and additive impact of BP control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005; 16: 3027-3037.