Department of Immunology and Nephrology, University Hospital Virgen Arrixaca, Spain
*Corresponding author: Manuel Muro, Department of Immunology and Nephrology, University Hospital Virgen Arrixaca, Murcia 30120, Spain
Received: October 16, 2014; Accepted: November 17, 2014; Published: November 17, 2014
Citation: Lopez-Hernandez R, Llorente S, Eguia J, Lopez M, Galian JA, et al. All that Glitter is not Gold: Persistent and Strong De Novo Anti-DSA Antibodies at High Titer Could not be Associated with AMR Whether Solid Phase Luminex SA C1q-Binding Assay is Negative in Post-Transplantation. Austin J Nephrol Hypertens. 2014;1(5): 1024.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964
Background: The complement-fixing ability of HLA antibody, irrespective of IgG (median fluorescence intensity) MFI strength could be a key component of clinical outcome. Recently, it has been developed a C1q-SAB assay that identifies complement-fixing HLA antibodies with high sensitivity and specificity. However, there is no consensus and each center determines its own threshold consistent with its transplant practices. This work presents an interesting case of de novo anti-DSA (donor specific antibody) antibodies development in the post-transplant period with a high titer (MFI>15000) in the routine SAB (single antigen beads) assay but not producing acute rejection and with result of C1qSAB being negative.
Case Presentation: A 39-year-old-man was transplanted with 2 HLA-A and 1 DR incompatibilities (recipient typing: A*30, *68; B*07. *35; DRB1*10, *14; DQB1*05, -; 2nd donor typing: A*02, *29; B*07, *35; DRB1*01, *13; DQB1*05, *06) (MMs in cursive) and with CDC cross-match negative. He was sensitized to HLA antigens by CDC and LMX (luminex) assays, with one previous transplant (14-years ago) (1st donor: A1, 3; B35, 57; DR7, -; DQ2, 9) (his SAB specificities against 1st donor were Bw4, A9, 25, 32, DR7, 9, 53, DQ2, 3). In the routine 2nd post-transplantation monitoring (9th month 2nd post-TX), we detected anti-2nd donor anti-DSA antibodies A2 (MFI=16000) and A29 (MFI=8000) by Luminex with our patient presenting an optima clinical situation. With these facts, nephrologists in our hospital indicated a renal biopsy which was completely normal, without any evidence of C4d deposits and the current creatinine of < 1.2 mg/dl. The rest of the post transplant course was uncomplicated. In spite of that, the patient was administered plasmapheresis and IVIG. We determined C1q SAB assay and the result was completely negative for A2 and A29 DSA alloantibodies. In C1qSAB assay, antibodies were assigned as ´posible´ when the first increase more than 33% (but at least 400 MFI) over the prior lower MFI bead was observed. Therefore, our patient had high titers of post-transplant DSA antibodies, but these antibodies did not seem to produce allograft injury and were not capable of fixing complement.
Conclusion: These data suggest that the determination of C1qSAB is very important to define the capability of anti-DSA antibodies to fixing complement in the post-transplantation period and clarify the treatment procedure.
Keywords: Fixing-complement; C1q; Luminex assay; Donor specific antibodies
HLA: Human Leucocyte Antigens; MFI: Median Fluorescence Intensity; SAB: Single Antigens Beads; DSA: Donor Specific Antibodies
Assessment of rejection risk based on preformed or de novo Donor Specific Antibody (DSA) is based on the Luminex (LMX) IgG Median Fluorescence Intensity (MFI) strength. Widespread debate exists over the suitable MFI cutoff value of Single Antigens Beads (SAB) for calling an antibody positive [1-3]. There is no consensus and each center determines its own threshold consistent with its transplant practices.
The complement-fixing ability of HLA antibody, irrespective of IgG (median fluorescence intensity) MFI strength could be a key component of clinical outcome. Recently, it has been developed a C1q-SAB assay that identifies complement-fixing HLA antibodies with high sensitivity and specificity. However, there is no consensus and each center determines its own threshold consistent with its transplant practices. C1q is the first step in the classical complement cascade activated by antibody and precedes C4d deposition. IgG1 and IgG3 activate complement and this response is triggered when C1q binds to the Cγ2 region of IgG1 and IgG3 at the Cμ3 region of IgM. Thus, we aimed to determine the correlation between IgG SAB and C1qSAB assays in patients in renal waiting list. This assay uses pooled luminescent beads, each uniquely distinguishable and coated with a different purified single HLA class antigen. This work presents an interesting case of de novo anti-DSA (donor specific antibody) antibodies development in the post-transplant period with a high titer (MFI>15000) in the routine SAB (single antigen beads) assay but not producing acute rejection and with result of C1qSAB being negative.
A 39-year-old-man was transplanted with 2 HLA-A and 1 DR incompatibilities (recipient typing: A*30, *68; B*07. *35; DRB1*10, *14; DQB1*05, -; 2ndd donor typing: A*02, *29; B*07, *35; DRB1*01, *13; DQB1*05, *06) (MMs in cursive) and with CDC cross-match negative. He was sensitized to HLA antigens by CDC and LMX (luminex) assays, with one previous transplant (14-years ago) (1st donor: A1, 3; B35, 57; DR7, -; DQ2, 9) (his SAB specificities against 1st donor were Bw4, A9, 25, 32, DR7, 9, 53, DQ2, 3). These luminex assays use pooled luminescent beads, each uniquely color code distinguishable and coated with a different purified single HLA class I antigen. Samples of immunized renal patients and negative and positive controls by LMX were used for validation, consisting of serum samples remaining from clinical testing of kidney transplantation. LMX-IgG assay was performed using SAB kits (LAB Screen, Onelambda, CA) according to the manufacturer and analyzed on a Luminex platform (LABScan100), as previously published [1,3]. Data were analyzed by Fusion 2.0 (OL, CA). Normalized MFI values from Fusion software were used to assign positive (´real´ MFI>1000) and ´possible´ (MFI=500-999). These assays detect all IgG binding antibodies irrespective of their complement-fixing ability.
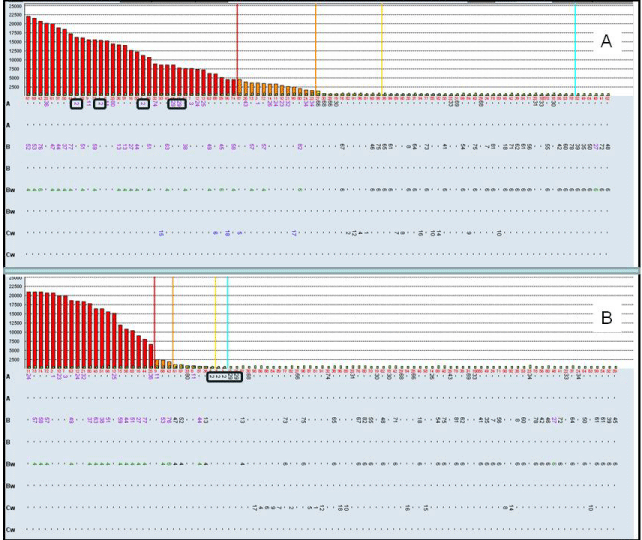
In the routine 2nd post-transplantation monitoring (9th month 2nd post-TX), we detected anti-2nd donor anti-DSA antibodies A2 (MFI=16000) and A29 (MFI=8000) by Luminex (Figure 1A) with our patient presenting an optima clinical situation. With these facts, nephrologists in our hospital indicated a renal biopsy which was completely normal, without any evidence of C4d deposits and the current creatinine of < 1.2 mg/dl. The rest of the post transplant course was uncomplicated. In spite of that, the patient was administered plasmapheresis and IVIG.
We also determined C1q SAB assay. Sera were inactivated by heating 30 min at 56°C. Five μl of serum were incubated with 5μl of LabScreen SAB for 20 min at Room Temperature (RT) and then incubated with 5μl of PE-conjugated sheep anti-human C1q for 20 min at RT, washed twice with 80μl LMX wash buffer and acquired on the LMX. The data were analyzed using ´Raw´ MFI values. In C1qSAB assay, antibodies were assigned as ´possible´ when the first increase more than 33% (but at least 400 MFI) over the prior lower MFI bead was observed. The C1q assay result was completely negative for A2 and A29 DSA alloantibodies (Figure 1B), corroborating the optimal clinical data. In conclusion, the determination of C1qSAB is very important to define the capability of anti-DSA antibodies to fixing complement in the post-transplantation period and clarify the treatment procedure.
In recent years, there has been increased interest in the study of anti-HLA antibodies involved in graft rejection because of the presence of C4d deposition in graft biopsy specimens, confirming injury to be due to anti-HLA antibodies. Previous studies have also shown that not all HLA-DSA have the same detrimental clinical impact. C1qSAB assay can detect IgM antibodies that can fix complement and several recent reports show that IgM DSA could be clinically relevant in absence of IgG antibodies. However, testing for IgM DSA is almost never performed. Using both LMX-IgG and LMX-C1q assays together gives more useful information. Indeed, C1q fixation and C4d deposition could not be directly correlated, as recently reported [4]. Detection of C1q gives the earliest indication of the potential for complement-mediated injury without a functional requirement for progression through the pathway to C4d deposition, which occurs later. Our patient had high titer of DSA antibodies but these anti-DSA antibodies had not capability to fixing-complement in C1q assay and these data were in accordance with clinical evolution. Thus, these data suggest that C1q assay could be an important method to evaluate the pre-transplant virtual cross-match and to define the non-permitted specificities (C1q-fixing) in kidney allograft transplantation. However, the lack of correlation between IgG MFI strength and the ability to fix complement provides a cautionary note [5-7]. The IgGSAB and C1qSAB together will now allow evaluation of the potential differential clinical impact of these distinct antibody populations on allograft outcome.
A) Luminex data with serum postransplant. The data correspond to single antigens HLA class I. DSA antibodies (A2 and A29) are very positive con MFImax=16000 B) Luminex data with the same serum postransplant analyzing C1q assay. DSA antibodies (A2 and A29) are negative. DSA antibodies are marked.