
Case Report
Austin J Nephrol Hypertens. 2023; 10(1): 1105.
Paradoxical Dapagliflozin Effect on Proteinuria in a Female Patient with Classic Fabry Disease First Literatura Report
Sebastián Jaurretche1,2*; Facundo Daminato3
¹Biophysics and Human Physiology, Medicine School, Instituto Universitario Italiano de Rosario, Santa Fe, Argentina
²Renal and páncreas transplant, Sanatorio Parque de Rosario, Santa Fe, Argentina
³Nephrology, Dialysis and Kidney Transplantation, Hospital Privado de Rosario, Santa Fe, Argentina
*Corresponding author: Jaurretche SInstituto Universitario Italiano de Rosario, Corrientes 2001, P.C: 2000. Rosario, Santa Fe, Argentina. Tel: 54-341-372841 Email: sebastianjaurretche@hotmail.com
Received: June 26, 2023 Accepted: July 31, 2023 Published: August 07, 2023
Abstract
A 75-year-old female patient, with “classic” Fabry disease (DEL 3&4 EXON, GLA gene) and severe multi-organic compromise (cardiac, brain, renal and peripheral nervous system) is presented. Patient presented treatment initiation criteria, and was a “classic” Fabry phenotype, for this reason, agalsidase-β was indicated. In addition, enalapril, were indicated. After the treatment start and to date, the patient presented: i) LVH stabilization; ii) brain damage stabilization; iii) stable eGFR and sustained improvement in proteinuria and; iv) pain improvement. In September 2022, due to the results of DAPA-CKD study, it was decided to start treatment with dapagliflozin to extend the renal protective effect; patient presented good tolerance to drug and no adverse effects were recorded. A slight decrease in eGFR was observed, but suspension was decided after 8 weeks due to increased proteinuria. Kidney disease is a major Fabry disease complication; it is more severe in the “classic” phenotype and males, although the “non-classic” Fabry phenotype and affected females may also present severe renal disease. Patients with Fabry disease and chronic kidney disease have a high risk of cardiovascular events, SGLT-2 inhibitor drugs have shown favorable effects on cardiovascular events in chronic kidney disease patients, both DBT and non-DBT. Multiple pathophysiological mechanisms have been described in Fabry nephropathy, none of them could explain, the reason why SGLT-2 blockade produced an increase in proteinuria in presented case.
Study limitation: short follow-up period of an isolated case.
Conclusion: SGLT-2 blockade effect should be analyzed in prospective studies, to define benefit or not in Fabry nephropathy.
Keywords: Fabry disease; Renal disease; Proteinuria; Dapagliflozin
Abbreviations: a-gal-A: a-Galactosidase A; CKD: Chronic Kidney Disease; DBT: Diabetes; ESRD: End-Stage Renal Disease; (DAPA) Dapagliflozin; eGFR: Estimated Glomefrular Filtration Rate; ERT: Enzyme Replacement Therapy; FD: Fabry Disease; LVH: Left Ventricular Hypertrophy; MNR: Magnetic Nuclear Resonance; PNS: Peripheral Nervous System; QST: Quantitative Sensory Testing; RAAS: Renin-Angiotensin-Aldosterone System; SGLT-2: Sodium-Glucose Co-Transporter 2; TGFP: Túbulo-Glomerular Feedback Pathway
Case Presentation
A 75-year-old female patient, with a history of heat and exercise intolerance, hypo-hidrosis, and recurrent febrile seizures accompanied by neuropathic pain in the extremities since childhood. FD was diagnosed by family screening, due to other affected patients in the family. The genetic test showed a pathogenic variant (DEL 3&4 EXON, GLA gene) causing the “classic” phenotype of Fabry disease (FD, OMIM #301500).
Complementary tests revealed severe organic involvement due to FD: i) cardiac: Left Ventricular Hypertrophy (LVH) on echocardiography and cardiac Magnetic Nuclear Resonance (MNR); ii) brain: brain MRN white matter lesions typical of FD; iii) renal: serum creatinine 0.8 mg/dl, estimated Glomefrular Filtration Rate (eGFR): 76.3 ml/min/m2, proteinuria: 800 mg/24 h and; iv) Peripheral Nervous System (PNS): Quantitative Sensory Testing (QST) with severe involvement of peripheral small fibers thin, also typical of FD. Cardiac, renal, brain, and PNS damage were attributed to DF after other causes had been ruled out. The GFR was estimated by the CKD-EPI equation and all determinations of urinary protein excretion were confirmed with a second sample. Since the moment of FD diagnosis, patient presented normal blood pressure, glycemia, and uric acid values during follow-up.
Patient presented treatment initiation criteria [10], and was a "classic" FD phenotype, for this reason, Enzyme Replacement Therapy (ERT) with agalsidase-β was indicated [1]. In addition, enalapril (at maximum tolerable doses), carbamazepine, acetylsalicylic acid, statins, and a cardio/nephro-protective diet were indicated.
After the treatment start and to date, the patient presented: i) LVH stabilization observed by echocardiography and cardiac MRN; ii) stabilization of brain damage: no new white matter lesions were observed in brain MRN; iii) stable eGFR (Figure 1) and sustained improvement in proteinuria (Figure 2) and; iv) pain improvement as evidenced by the SF-36 questionnaire.
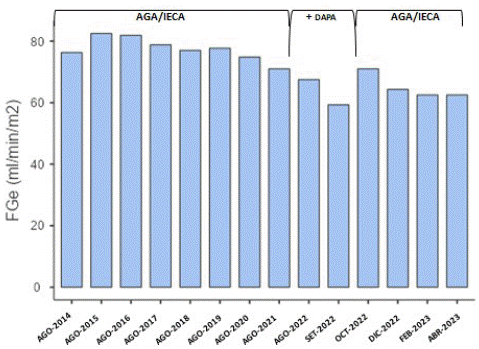
Figure 1: Estimated glomerular filtration rate during follow-up. References: AGA: Agalsidase-β; ACEI: Angiotensin Converting Enzyme Inhibitors; DAPA: Dapagliflozin

Figure 2: Urinary protein excretion during follow-up.
References: AGA: Agalsidase-β; ACEI: Angiotensin Converting Enzyme Inhibitors; DAPA: Dapagliflozin
In September 2022, due to the results of DAPA-CKD study [2], it was decided to start treatment with Dapagliflozin (DAPA) 10 mg/day to extend the renal protective effect; patient presented good tolerance to the drug and no adverse effects were recorded. A slight decrease in eGFR was observed, but suspension was decided after 8 weeks due to increased proteinuria (Figures 1 & 2).
Discussion and Conclusion
Fabry disease is a progressive, X-linked lysosomal storage disorder caused by GLA gene variants, which encodes the enzyme a-galactosidase A (a-gal-A; EC 3.2. 1.22) [3]. Deficient enzyme activity leads to intracellular accumulation of globotriaosylceramide and associated metabolites that are substrates for a-gal-A [3]. Pathological and progressive deposition of globotriaosylceramide occurs in all types of kidney cells and causes renal dysfunction [3].
Kidney disease is a major complication of FD [4-6]; it is more prevalent and severe in the “classic” phenotype and in men [3,4], although the “non-classic” FD phenotype and affected women may also present severe renal disease [3,7].
Renal damage due to Fabry is characterized by the presentation of proteinuria and decreased Glomerular Filtration Rate (GFR) [4,5]. The eGFR progressive decrease is directly related to the degree of proteinuria and, in FD natural history, it is more pronounced in patients with an initial GFR < than 60 ml/min/1.73m2 [4-6]. Chronic Kidney Disease (CKD) caused by FD culminates in End-Stage Renal Disease (ESRD) requiring renal function replacement therapy around the age of 40 in men affected by the FD "classic" phenotype [3-6]. Patients with FD and CKD have a high risk of cardiovascular events, similar to patients with CKD of any cause [4-6]. ERT with recombinant human agalsidase and pharmacological chaperones are specific therapies for FD nephropathy [8,9], but pharmacological and non-pharmacological nephroprotective interventions are also necessary to prevent the progression of FD kidney damage [3-6]. Blockade of the Renin-Angiotensin-Aldosterone System (RAAS) has been the first-line pharmacological strategy to eGFR slow decline in CKD of various causes, including FD [10]. Sodium-Glucose Co-Transporter 2 (SGLT-2) inhibitor drugs (SGLT-2i) have recently shown favorable effects on cardiovascular events in CKD patients, both DBT and non-DBT [2]; therefore, SGLT-2i are additional drugs to RAAS inhibitors to extend renal protection in CKD. We present a case of a non-diabetic woman with FD nephropathy who had previously received treatment with agalsidase-β and enalapril, who was prescribed DAPA as a nephroprotective effect.
Clinical case of an elderly woman affected by the "classic" FD phenotype, the most severe clinical form of the disease, is presented. The patient had heart, brain, kidney and PNS damage, organs typically affected by "classic" FD. Stabilization of cardiac and brain damage added to the improvement of PNS clinical manifestations was previously described in the "case presentation" section and has been maintained to date with the indicated therapeutic interventions. The evolution of FD kidney damage will be discussed separately, as it is the purpose of this work.
At time of FD diagnosis, our patient had an eGFR: 76.3 ml/min/m2 and proteinuria: 800 mg/24 Hs. It has been described that renal damage occurs prior to FD diagnosis in 61% of affected patients [11]. In reports of the natural history (treatment naive) of nephropathy due to DF, it was observed that all affected meles have an annual eGFR loss greater than the general population, while among some FD females the annual eGFR decline is greater than the general population [12]; in all cases, proteinuria is a high prognostic indicator of eGFR faster decline [12]. The patient described in this work presented poor renal prognosis indicators at the time of diagnosis, which implies a rapid loss of renal function without treatment; however, during the first seven years of follow-up, the annual eGFR loss was 0.9 ml/min/m2 and significant decrease in proteinuria, receiving agalsidase-β and RAAS inhibitor drugs was observed. This therapeutic response is consistent with results of Jain et al., in 2011 and Ortiz et al., in 2021, who reported that agalsidase-β in plus RAAS inhibitors are capable of modifying the FD nephropathy natural history at any CKD stage, even in patients with FD “classic” severely affected [10,13].
The mechanisms and glomerular effects by which SGLT-2i produce an initial eGFR decrease and proteinuria improvement, achieving nephroprotection in DBT and non-DBT patients with CKD, are now widely known, from the results of DAPA-CKD study [2]. The main determinant of the initial eGFR drop produced by DAPA is due to modification of Túbulo-Glomerular Feedback Pathway (TGFP); the increased natriuria in tubular lumen due to the blockade of the secondary active co-transport SGLT-2, causes (via TGFP) a glomerular afferent arteriole vasodilatador effect, which decreases the glomerular capillary hydrostatic pressure and therefore, lower filtration pressure, primary beneficial effect in preventing CKD progression [2]. However, this is not the only variable that modifies glomerular hemodynamics [2,14] and various causes of CKD may probably, affect it in different ways [14].
Heerspink et al. analyzed the variables that influence the initial eGFR drop produced in non-DBT patients receiving DAPA [15]. In this study, authors included a FD patient, among other causes of nephropathy, although this case is not analyzed individually. Their univariate analysis reported that age, blood urea and creatinine prior to starting DAPA are variables directly related to the magnitude of the initial eGFR decrease produced by DAPA; while initial eGFR and Hemoglobin are inversely related variables [15]. In their multivariate analysis, they also demonstrated that eGFR prior to starting DAPA treatment is an independent determinant of the initial eGFR decrease produced after starting DAPA in non-DBT patients [15]. In their results, they reported that patients with CKD stage 3 (according to KDIGO) present a greater drop in eGFR than patients in stage 4 and 5 [15]. The patient in our clinical case was in KDIGO stage G2A3.
When non-DBT CKD patients are classified according to eGFR and proteinuria, different responses to DAPA are observed:
- “Hyper-filtration” group: increased eGFR and increased proteinuria
- “Glomerular injury” group: decreased eGFR and increased proteinuria (the case of our patient)
- “Non-progressors” group: increased eGFR and decreased proteinuria
- “Glomerular collapse” group: decreased eGFR and decreased proteinuria
The patients in the “hyper-filtration” group show greater drops in GFR after the DAPA start treatment, logically, due to the pre-existing glomerular hemodynamics, which is modified with SGLT2 blockade. “Glomerular injury” patientes group, as in our case, present slight eGFR decreases with DAPA [15].
In CKD animal models of hypertensive cause, it has been described that SGLT-2 can be over-expressed, which can attenuate the eGFR drop produced by DAPA treatment [16]. This mechanism has not been studied in FD nephropathy.
The eGFR slight drop with DAPA treatment presented in our patient could be explained by what was previously stated, although the proteinuria increase is an unexpected finding. SGLT-2 blockade produces a proteinuria decrease in patients with DBT and non-DBT CKD [15,17-19], even the effect on proteinuria has been correlated to the eGFR slope and duration of eGFR drop produced by DAPA as reported by experts [15].
Multiple pathophysiological pathways and mechanisms have been described in FD nephropathy, none of them could explain, even hypothetically, the reason why SGLT-2 blockade produced an increase in proteinuria in the case we present, for this reason it was written the present work. The present study limitation is the short follow-up period of an isolated case, therefore, as a conclusion, the SGLT-2i effect should be analyzed in prospective controlled studies with a larger number of patients, to define the benefit or not of SGLT-2 blockade on CKD caused by FD. The SGLT-2i effect will be analyzed in FD patients and CKD stage 1 to 3 in a prospective controlled study, the design of this work was recently published [20]. We present the first case in the literature.
References
- Germain DP, Altarescu G, Barriales-Villa R, Mignani R, Pawlaczyk K, Pieruzzi F, et al. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. 2022; 137: 49-61.
- Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021; 9: 755-66.
- Germain DP. Fabry disease. Orphanet J Rare Dis. 2010; 5: 1-49.
- Jaurretche S, Antogiovanni N, Perretta F. Prevalence of chronic kidney disease in Fabry disease patients: multicenter cross sectional study in Argentina. Mol Genet Metab Rep. 2017; 12: 41-3.
- Jaurretche S, Antongiovanni N, Perretta F. Fabry nephropathy. Role of nephrologist and clinical variables associated with the diagnosis. Nefrologia (Engl Ed). 2019; 39: 294-300.
- Jaurretche S, Antongiovanni N, Perretta F. Vascular disease in male patients with Fabry disease on hemodialysis: a retrospective cohort study in Argentina. Rev Nefrol Dial Transpl. 2019; 39: 101-7.
- Perretta F, Antongiovanni N, Jaurretche S. Major organic involvement in women with Fabry disease in Argentina. ScientificWorldJournal. 2018; 2018: 6515613.
- Tøndel C, Thurberg BL, DasMahapatra P, Lyn N, Maski M, Batista JL, et al. Clinical relevance of globotriaosylceramide accumulation in Fabry disease and the effect of agalsidase beta in affected tissues. Mol Genet Metab. 2022; 137: 328-41.
- Jaurretche S. Chaperonas farmacológicas. Nueva alternativa terapéutica para la nefropatía por enfermedad de Fabry en Argentina. Rev Nefrol Dial Transpl. 2020; 40: 51-61.
- Jain G, Warnock DG. Blood pressure, proteinuria and nephropathy in Fabry disease. Nephron Clin Pract. 2011; 118: c43-8.
- Jaurretche S, Antongiovanni N, Perretta F. Prevalence of renal damage previous to the diagnosis of Fabry disease in Argentina. Rev Nefrol Dial Traspl. 2022; 42: 1-10.
- Wanner C, Oliveira JP, Ortiz A, Mauer M, Germain DP, Linthorst GE, et al. Prognostic indicators of renal disease progression in adults with Fabry disease: natural history data from the Fabry registry. Clin J Am Soc Nephrol. 2010; 5: 2220-8.
- Ortiz A, Kanters S, Hamed A, DasMahapatra P, Poggio E, Maski M, et al. Agalsidase beta treatment slows estimated glomerular filtration rate loss in classic Fabry disease patients: results from an individual patient data meta-analysis. Clin Kidney J. 2021; 14: 1136-46.
- Hall JE. Guyton & Hall. Tratado de fisiología médica. Elsevier Health Sciences. 2021.
- Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021; 42: 1216-27.
- Miyata KN, Lo CS, Zhao S, Liao MC, Pang Y, Chang SY, et al. Angiotensin II up-regulates sodium-glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice. Clin Sci (Lond). 2021; 135: 943-61.
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375: 323-34.
- Piperidou A, Sarafidis P, Boutou A, Thomopoulos C, Loutradis C, Alexandrou ME, et al. The effect of SGLT-2 inhibitors on albuminuria and proteinuria in diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019; 37: 1334-43.
- Sternlicht HK, Bakris GL. Reductions in albuminuria with SGLT2 inhibitors: a marker for improved renal outcomes in patients without diabetes? Lancet Diabetes Endocrinol. 2020; 8: 553-5.
- Battaglia Y, Bulighin F, Zerbinati L, Vitturi N, Marchi G, Carraro G. Dapaglifozin on albuminuria in chronic kidney disease patients with FabrY disease: the DEFY study design and protocol. J Clin Med. 2023; 12: 3689.