
Review Article
Austin J Nephrol Hypertens. 2015;2(1): 1031.
Sexual Dysfunction in Dialysis Patients: A Review
Raza Mustafa* and Rebecca J Schmidt
Department of Medicine, Division of Nephrology and Hypertension, West Virginia University, USA
*Corresponding author: Raza Mustafa, Department of Medicine, Division of Nephrology and Hypertension, West Virginia University, Morgantown
Received: November 07, 2014; Accepted: January 28, 2015; Published: January 30, 2015
Abstract
Normal sexual function is an important component of quality of life. Sexual dysfunction is common in patients with Chronic Kidney Disease (CKD), especially those with End Stage Renal Disease (ESRD) and sufficiently reduced kidney function to require renal replacement therapy. The symptoms of SD start early with declining Glomerular Filtration Rate (GFR) and are rarely improved after starting dialysis treatment. Erectile dysfunction in men and anovulation in women are the most common symptoms. The etiology of these symptoms is complex and multifactorial. While phosphodiestrase - 5 inhibitors are effective in men with erectile dysfunction, no effective therapy exists for women with SD and women on hemodialysis are generally counseled to avoid pregnancy. Renal transplantation is considered the best option for women of childbearing age planning to conceive. The following review is focused on the physiology SD in CKD and available treatment options available.
Keywords: Chronic kidney disease (CKD); Sexual dysfunction (SD)
Introduction
Quality of life (Qol) has emerged as an important outcome measure in patients with chronic illnesses. The impact of chronic diseases on Qol is formidable but traditionally has focused on factors that are physical rather than those contributing to emotional and mental well-being. In the general population, physical Qol begins to decline in the fifth decade of life. Chronic diseases such as diabetes, coronary artery disease and chronic kidney disease (CKD) are known to accelerate this decline and, additionally, impact mental and emotional Qol. Sexual dysfunction (SD) is a common problem in patients with CKD and can significantly impact Qol.
Impaired sexual function can have damaging effects on selfconfidence, sense of wholeness, social and the marital relationship [1]. In a report from National Health and Social Life Survey (NHSLS), theprevalence of SD in general population was 43% and 31% in women and men, respectively, and SD was associated with lower Qol [2]. Diabetes mellitus, one of the main causes of CKD, have been associated with SD in both men and women [3]. Sexual dysfunction is common in men with CKD, with approximately 70% reporting erectile dysfunction, a percentage much higher than general population [4].
Little information exists about how patients with End-Stage Renal Disease (ESRD) cope with quality of life issues such as SD. Multiple co-morbid conditions are common in patients with ESRD including diabetes and cardiovascular disease as well as other debilitating conditions such as impaired vision, deafness, poor mobility, arthritis and cognitive dysfunction. Sexual dysfunction is also prevalent in this population. This review will cover some of the physiologic and social barriers to improve outcomes of SD in patients with CKD and ESRD.
Altered Reproductive Physiology in Men with Chronic Kidney Disease
Moderate reductions in glomerular filtration rate (GFR) result in disturbance of the pituitary – gonadal axis [5]. Uremia affectslocal amino acid neurotransmitter outflow in hypothalamus affecting the pulsatile release of gonadotropin releasing hormone (GnRH), leading to a hypogonadal state [6]. Total and free testosterone levels are typically reduced in CKD [5] and stimulation response of testosterone secretion by GnRH produces only a blunted response. Serum estradiol and total estrogens levels are usually elevated [6] Plasma levels of LH are elevated presumably due to prolonged halflife and diminished secretion of testosterone from Leydig cells [7]. Because plasma FSH levels are variably elevated, the ratio of LH/FSH generally remains normal. Serum prolactin levels are also increased in men on hemodialysis and are not suppressed by external dopamine infusion.
Chronic kidney disease is associated with decreased spermatogenesis and testicular damage, resulting in a decreased volume of ejaculate and low percentage of sperm motility. Interstitial fibrosis and calcifications can develop in the epididymis and corpora cavernoasa; particularly as time on hemodialysis becomes prolonged [8]. These abnormalities persist throughout the continuum of CKD and are not altered by dialytic therapy.
Altered Reproductive Physiology in Women with Chronic Kidney Disease
Follicle stimulating hormone and LH release are decreased in women [7]. However, it remains unclear whether the disturbance is in the hypothalamus (due to decrease GnRH release) or in anterior pituitary [5]. Pre-ovulatory LH and estradiol peaks are frequently absent. Circulating prolactin levels are also increased. In postmenopausal women with CKD, GnRH levels are higher compared to the women of similar age with normal GFR.
Menstrual cycles are irregular with scanty flow after the initiation of maintenance dialysis. Some women may experience hypermenorrhagia leading to significant blood loss, but anovulatory cycles are the rule in women with reduced renal function. There is an absence of progesterone effects and failure to increase the body temperature at the time of expected ovulation [5].
Clinical Manifestations of Sexual Dysfunction in Men
Erectile dysfunction (ED) is the most often reported symptom of SD in dialysis patients with reported rates as high as 90% in older patients. A study of 390 prevalent hemodialysis reported a prevalence of 82% in all HD subjects [4]. Forty-five percent of men had severe ED according to Sexual Health Inventory for Men (SHIM) questionnaire. Younger patients aged less than 50 also demonstrated a high prevalence of ED (63%). Another study [9] of dialysis patients with mean age of 49.3 years found a prevalence rate of 80.7% (Figure 1).
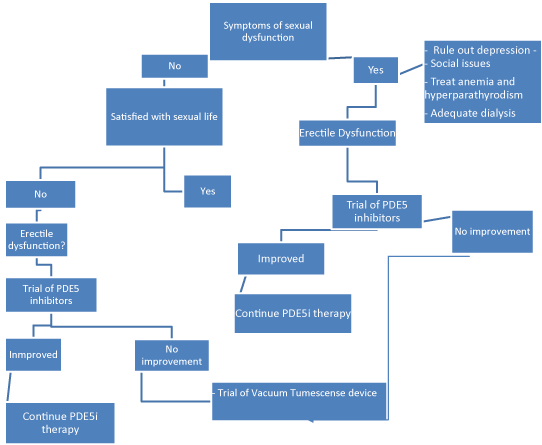
Figure 1: Treatment algorithm for men with sexual dysfunction.
ED= erectile dysfunction, PDE5I: Phosphodiesterase 5 inhibitors
Decreased libido is reported in about 86% of patients on hemodialysis [10] compared to 8% normal subjects. Similarly, delayed or lack of ejaculation was described in 52% of hemodialysis subjects compared to 5% normal subjects. Rate of premature ejaculation, however, were similar in both groups. Oligospermia and decreased sperm motility is also common.
Gynecomastia occurs in approximately 30% of men on maintenance hemodialysis [11]. This problem develops most often in the initial months of dialysis and tends to regress as dialysis continues. The pathogenesis is unclear, but elevated prolactin and increased estrogen-androgen ratio seem the most likely etiology. Increasing use of antiandrogenic medication (such as spironolactone) in the CKD population may be an etiologic factor in incident dialysis patients.
Depression and anxiety may be the presenting symptoms of SD. The prevalence of depression may be as high as 80.5% in dialyzed women and 72.7% in dialyzed men [12]. A satisfactory sexual life makes the patient feel less anxious and depressive and evaluate his/ her status of general health more favorably.
Clinical Manifestations of Sexual Dysfunction in Women
The symptoms of SD in women include lack of desire and arousal, decreased vaginal lubrication, lack of satisfaction and pain with sexual intercourse. Factors associated with likelihood of having symptoms include increasing age, presence of diabetes, cardiovascular disease, and presence of depression [13]. Women who had experienced a sad situation within the last year (such as death of a loved one), postmenopausal women, women on hemodialysis, medication use, delivery of three or more live infants, or previous abdominal surgery have been cited as risk factors for increased SD symptoms [13]. In contrast, adequacy of dialysis, duration of dialysis, time on dialysis, serum albumin, and hematocrit levels were not found pose risk factor for SD (Figure 2) [13,14].
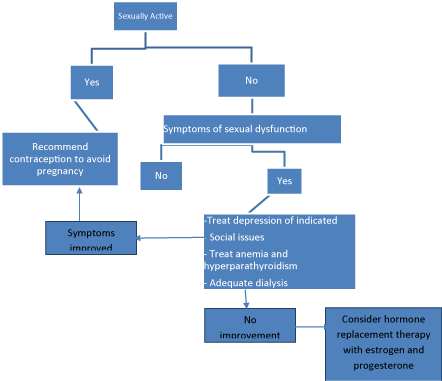
Figure 2: Treatment algorithm for women with SD.
Disturbance in menstruation and fertility are common in women with CKD, leading to amenorrhea by the time patients are started on dialysis. Menstrual cycles remain irregular, and are anovulatory resulting in infertility. Although rare, pregnancy can occur in women with advanced CKD, but the chances for fetal wastage are high and patients may require more intensive renal replacement therapy [15].
Pathophysiology of SD
The etiology of SD symptoms in dialysis patients is complex and includes physiological, psychological and iatrogenic factors. Atherosclerosis is a wide spread phenomenon in dialysis patients and is accelerated by comorbid conditions common in this population (diabetes mellitus, hypertension, secondary hyperparathyroidism, inflammation) as well as the impact of uremic toxins on the vascular endothelium. Calcification of the cavernous artery was found in 78% of dialyzed patients [8]. Decreased penile arterial flow rate results in cavernous insufficiency and ED [16]. A decrease in insulin-like growth factor 1 (IGF-1) [17]. can cause deterioration of vascular muscle cells and contribute to ED. Anemia and pulmonary microembolization can cause tissue hypoxia which leads to diminished synthesis of the body’s most potent vasodilator, nitric oxide [18]. Sensory and motor neuropathy may contribute to the symptoms of SD, especially those with diabetes. Hyperprolactinemia in CKD causes ED, infertility and decreased sexual appetite in both men and women. Low testosterone levels also contribute to the deterioration in erectile function.
Depressive episodes and marital conflicts are more frequent in dialyzed patients as compared with the general population, and often linked with worsening socioeconomic situation. A strong correlation between stress, depression and SD in the general population is well recognized. Depression has long been accepted as the most common psychiatric illness in renal failure patients [19].
Pharmacologic treatment of conditions associated with CKD may also significantly affect sexual function. Beta- adrenergic antagonists, diuretics, vasodilators, digoxin, fibrates and antidepressants can contribute to the sexual dysfunction in CKD and dialysis patients
Treatment Options for Sexual Dysfunction
Therapies to treat sexual dysfunction are limited. Since ED is the most common complaint of SD by male hemodialysis patients, most of the treatment strategies are targeted against this complaint. This may also have been propelled by popularity of phosphodiesterase -5 inhibitors (PDE5i) showing effectiveness in general population with ED. Some of the therapeutic options studied in hemodialysis population are described below.
Phosphodiesterase – 5 inhibitors (PDE5i)
Phosphodiesterase -5 inhibitors (PDE5i) act by increasing intracavernoal cyclic guanosine monophosphate (cGMP) levels, leading to prolonged erectile periods. Four compounds are available: Sildenafil (50-100 mg, taken an hour before sexual activity) , Vardenafil (Similar to Sildenafil) , Tadalafil (10-20 mg, effects can last upto 36 hours )and Avanafil (50-200 mg, short duration of action). All compounds are equally effective, but tadalafil has the longest duration of action. In a randomized, double-blind, placebo controlled study of oral sildenafil (50 mg) in hemodialysis patients showed improvement in International Index of Erectile Functions(IIEF) score [20]. Erectile frequency, erection quality, penetration ability, maintenance frequency of penetration and erection confidence score was increased. There was no increase in sexual desire. Eighty-five percent of patients reported improvement with Sildenafil compared to 9.5 % in placebo group. However, there were only 20 patients in study group and follow up was only for one month. Sildenafil was well tolerated with no increase in adverse reactions compared to placebo. Similar results have been reported in peritoneal dialysis (PD) patients also. An 8-week, prospective, double blinded, placebo controlled, cross over study randomized 16 patients undergoing PD to receive 4 weeks of either placebo or Sildenafil (50 mg initially, increased to 100 mg if tolerated [21]. Sildenafil treatment significantly improved International Index of Erectile Function (IIEF) scores compared with baseline and placebo. Seventy-five percent of sildenafil treated patients reported improved erection scores. When compared head to head, Sildenafil and Vardenafil produced similar improvement in ED and health-related Qol in hemodialysis patients [22].
Other open-label studies and case series indicate that sildenafil is effective and well tolerated in patients undergoing dialysis. A list of these reports is summarized in (Table 1-3) [23].
MEN
WOMEN
- Decreased levels of serum GnRH
- Decreased serum testosterone levels
- Increased serum estradiol and total estrogens levels
- Increased serum LH levels
- Normal to elevated serum FSH levels
- Normal FSH/LH ratio
- Increased serum prolactin levels
- Decreased serum GnRH levels
- Decreased serum FSH and LH levels
- Low serum estradiol levels
- Low serum progesterone levels
- Loss of pulsatile secretion of LH
- Increased serum prolactin levels
GnRH= Gonadotropin Releasing Hormone, FSH= Follicle Stimulating Hormone, LH= Luteinizing Hormone
Table 1: Endocrine changes with kidney disease.
Hormones: Androgens, estrogens, progesterone
Beta blockers: Atenolol, pindolol, propranolol
Vasodilators: Hydralazine
Diuretics: Thiazides, spironolactone
Sympatholytics: Clonidine, methyldopa, reserpine
Sedatives: Barbiturates
Psychotropic drugs: Tricyclic antidepressants, MAO inhibitors
Others: Digoxin, ranitidine
Table 2: Commonly used drugs associated with erectile dysfunction in men with CKD (74).
Study
N
Study Type
ED duration Mean
Time on dialysis Mean
PDE5i and dose
Efficacy end point (s)
Jacques and Abrairs, 1999 [28]
4
Case series
NR
2 months – 6 years
50 mg of sildenafil (varying intervals)
IIEF improvements in all patients
Chen et al., 2001
[29]
35
Open label
44.2 months (18-120)
61.6 months (24-44)
25,50 or 100 mg of Sildenafil PRN
80% had IIEF score improvement, 77% satisfied with EF, 71 % of partners satisfied with treatment
Puzo et al., 2001 [30]
18
Open label
NR
NR
25 or 50 mg of Sildenafil
Improvement in sexual activity and desire
Rosas et al., 2001 [31]
15
Open label
67% had ED> 1 year
3.9+- 3.5 years
25, 50 or 100 mg of sildenafil
Significant improvement in IIEF domains of EF, Orgasmic function and intercourse satisfaction
Turk et al., 2001 [32]
37
Open label
HD: 25 +- 27 months
PD: 16+- 16 months
35+- 29 months
50 or 100 mg of sildenafil
Significantly improved IIEF scores, both in HD and PD
Seibel et al., 2002 [20]
41
DBPC
NR
36 ± 26 months placebo, 42 ± 31 months of Sildenafil
50 mg of Sildenafil prn
Significantly improved IIEF scores vs baseline and vs placebo. All domain score improved except sexual desire, 85 % improved EF
Yenicerio Glu et al., 2002 [33]
41
Open label
HD: 2.2 ± 2.0 years, PD: 1.4 ± 0.6 years
Hd: 4.4 ± 2.5 years, PD: 1.6 ± 1.4 years
25 mg of Sildenafil
Significantly improved total IIEF scores (HD and PD)
Significant improvement in Fugl-Meyer life satisfaction scale
Hyodo et al., 2004 [34]
14
Open label
NR
3.1 ± 3.8
25 or 50 mg of Sildenafil
57.1% of patients improved IIEF scores to be > 20
Sahin et al., 2004 [35]
51
Open label
4.7 years (6-10 years)
58.2 ± 42.3 months (1-179 months)
Single dose of Sildenafil 50 mg
Overall response rate of 74.5 % on IIEF –EF
Mahon et al., 2005[21]
16
Randomized, placebo-controlled crossover
6 months – 7 years
6 months – 5 years (PD)
50 mg of Sildenafil PRN initially for 2 weeks then 100 mg
Significant improvement vs. baseline and placebo in IIEF domains of EF, intercourse satisfaction and overall satisfaction
Dachille et al., 2006 [36]
42
Open label
84 months ( 7-228 months)
5 months
50 mg of Sildenafil PRN for one week, 25, 50, or 100 mg of Sildenafil for 2 weeks
97 % response rate to sildenafil
Tas et al., 2006 [37]
16
Open label
NR
NR
Epo + 25, 50 or 100 mg of sildenafil PRN
Significantly improved IIEF score, significant improvement in erection frequency, firmness, maintenance ability, and confidence, intercourse enjoyment, desire levels and relationship satisfaction
Turk et al., 2010 [22]
32
Randomized head –t- head crossover
>6months
45.9 ± 48.2 months
50 mg of Sildenafil or 10 of Vardenafil once a week
Significantly improved IIEF-5 and SF-36 scores vs. baseline
Ghafari et al., 2010 [38]
27
DBPC
NR
6 months – 11 years
50-150 mg of Sildenafil PRN
Significantly improved IIEF-5 score vs. placebo in all domains
DBPC: Double-Blind; Placebo-Controlled; ED: Erectile Dysfunction; GA: Global Assessment; HD: Hemodialysis; IIEF: International Index of Erectile Function; NR: Not Reported; PD: Peritoneal Dialysis; PRN: Taken as Needed; SF-36: Medical Outcome Study 36 –item Short –Form Health Survey
Table 3: Laboratory values at hospital admission.
Pharmacokinetics of PDE5 inhibitors
In men with mild or moderate renal impairment, sildenafil pharmacokinetics were not significantly altered. It is rapidly absorbed and 96 percent of the drug is bound to plasma proteins [24]. It is primarily metabolized by cytochrome P450 (CYP) enzyme 3A4 and 2C9. Sildenafil metabolites are excreted predominantly in feces (80%), although a small amount is excreted in urine (~13%) [25]. Sildenafil clearance was significantly reduced when creatinine clearance was less than 30 ml/min (23). Hemodialysis does not seem to clear sildenafil or its major metabolite (UK-103,320) [24]. The recommended starting dose of sildenafil in patients with CrCL <30 ml/min is 25 mg [26]. No dosage adjustments are required for vardenafil in renal insufficiency, however, the prescribing information warns that vardenafil has not been studied in patients requiring dialysis [27]. Pharmacokinetics of Tadalafil and Vardenafil are similar to Sildenafil. A lower starting dose is recommended for patients with CrCl < 30 ml/min or on hemodialysis.
Adverse effects are generally mild and may include headaches, facial flushing and dyspepsia and their frequency is similar to men without kidney disease and there are no reports of priapism or chest pain. However, since hypertension, hypotension, ventricular arrhythmia, palpitations and angina are more common in ESRD population, patients should be warned about possible changes in cardiac function [23]. Overall, PDE5i are effective for patients with ED on hemodialysis and should be considered as a first line agent for treatment
Testosterone
Testosterone deficiency is common in patients with kidney disease and has been associated with poor clinical outcomes [39,40]. In one report, testosterone deficiency (< 10 nmol/L) was found in 44% of ESRD men while 33% showed testosterone insufficiency (10- 14 nmol/L). Only 23% of men had normal testosterone levels (>14 nmol/L) [41]. Testosterone supplementation for ED is effective for primary hypogonadism. A case series of 27 patients with ESRD and biochemical hypogonadism were given testosterone enanthate (500 mg) IM every 3 weeks until their biochemical hypogonadism was corrected [42]. Only 3 patients (11.1%) restored sexual function completely, 19 (70.3%) had partial response varying from an increased sense of well-being alone to restored sexual function. Five patients (18.5%) had no response. Vacuum tumescence devices were offered to non-responders and 73.1% of patients had full correction of ED, suggesting that testosterone alone may not be adequate to restore the sexual dysfunction in hypogonadal male on dialysis. In a pilot study of testosterone gel treatment for ED (5 mg per 24 h), hemodialysis patients with biochemical hypogonadism reported significant improvement in IIEF score over one month [43]. These patients had low serum testosterone levels (bioavailable serum testosterone levels < 3.82 nm/L). No significant side effects were reported though the hematocrit increased by 4.9% at 6 months. Another small case series of 4 dialysis patients and 8 renal transplant patients reported that combined intramuscular injection of testosterone and oral sildenafil improved IIEF score in patients with low testicular volume [44]. However, it was not reported in the study if combined treatment was better than testosterone or sildenafil alone. In the absence of randomized trials and lack of symptom improvement, testosterone cannot be recommended as single agent therapy for SD. Therapy with combination of testosterone and PED5i warrants further investigation, especially in patients who have significantly low serum testosterone levels
\Zinc therapy
Zinc deficiency in experimental animals and males during the growing age causes growth retardation and testicular atrophy [45,46]. While oral zinc can increase the testosterone levels [47] the effect of zinc on SD is not clear. A small, placebo controlled, double blind trial randomized 20 patients on hemodialysis to receive oral zinc (50 mg of elemental zinc daily) or placebo [48]. At the end of the 6 month, a significant increase in plasma zinc, serum testosterone, and sperm count occurred in zinc treated group. Patients receiving zinc reported improved libido and frequency of intercourse. However, another randomized study failed to confirm the effects of zinc on sexual dysfunction [49]. Overall, there is no large, well designed trial to make recommendations about zinc therapy for SD.
Vacuum tumescence device
A vacuum tumescence device may be effective in restoring potency in males with ED who are unresponsive to medical therapy. The device is a plastic cylinder with an aperture at one end that is placed over the penile shaft; at the other end of the cylinder is a pump mechanism that is used to generate negative pressure within the cylinder; the negative pressure causes an inflow of blood into the corporal bodies thereby leading to erection.In one report, [42]. the device completely corrected penile dysfunction in 19 of 26 impotent patients, after aninadequate response to treatment with testosterone for hypogonadism. Vacuum devices alone have not tested in dialysis patients.
Other Therapies
Alprostadil
Intraurethral administration of prostaglandin E1, alprostadil results in erection sufficient for intercourse. However, the efficacy and safety of this medication for ED has not been tested in patients with CKD.
Treatment of Sexual Dysfunction in Women
A paucity of studies exists concerning the therapy of decreased libido and sexual function in women with CKD and ESRD receiving hemodialysis despite prevalence rates of 80% or higher for SD [50]. Psychosocial issues and lack of interest are common barriers [51]. Depression is significantly more prevalent in female dialysis patients compared to men [52]. Contraceptive methods are recommended for women of child bearing age who are on dialysis and having regular menstrual periods.
Patients with vaginal atrophy and dryness may benefit from local estrogen therapy or vaginal lubricants. Hormone replacement therapy may restore regular menstruation. In a 12 month study,premenopausal women on dialysis, treated with estradiol and cyclic noretisterone acetate reported increased sexual activity and libido [53]. The estrogen-progestin therapy induced regular menses and women in treatment group reported increase in physical wellbeing and activity, mood, mental alertness and self-respect. The choice of such therapy, however, must be weighed against the cardiovascular risk associated with hormone replacement therapy.
Restoration of fertility as a therapeutic goal should be discouraged in women on dialysis. Abnormalities of ovulation can usually be reversed by successful renal transplantation as menstruation resumes in many patients [53]. Symptoms of SD as measured by female sexual function index (FSFI) scoring, may also improve after renal transplantation. [45].
Treatment of Comorbid Conditions
Other medical conditions frequently attendant to ESRD such as anemia, depression and hyperparathyroidism have also been implicated as contributory to sexual dysfunction. The use of erythropoietin may positively impact SD [54]. One study suggested a link between increased gonadotropin levels and improved SD in dialysis men given erythropoietin therapy [55], while others reported a decrease in prolactin levels [56]. It is unclear if erythropoietin is directly responsible for changes in hormone levels.
The presence of depressive symptoms are highly prevalent and are an independent risk factor for SD. In a comprehensive approach to the management of SD, a thorough evaluation of psychological depression must be included [57]. Antidepressant medications may also be helpful.
Weisboard and colleagues [58] conducted a well-designed, unblended, randomized control trial in hemodialysis patients comparing two management strategies for pain, ED and depression. They randomized 220 hemodialysis patients to 12 months of either “feedback” or “nurse management”. In both groups, symptoms were assessed on monthly bases. In the feedback group, the patient’s renal provider received a letter describing his or her patient’s symptoms along with algorithms that could be used to guide treatment. Any treatment was left to the provider’’s discretion. In the nurse management group, trained nurses evaluated patients, provided recommendations for treatment directly to renal providers and facilitated the implementations of those treatment recommendations. Nurse management did not provide additional benefit compared with feedback; however, both groups experienced small, statistically significant improvements in symptoms from their “usual care” baseline.
Although a causal link between SD and secondary hyperparathyroidism has not been demonstrated, a positive impact on SD with therapy has been suggested. In one study, sexual function increased three months after parathyroidectomy [59]. The effect of medical treatment for mineral metabolism abnormalities of CKD, including the role of 1,25dihydrocholecalciferol is not clear [60].
A relationship between the modality of dialysis and the development of SD is not clear. A post-hoc analysis of randomized controlled trial showed no improvement in SD in patients undergoing frequent nocturnal dialysis (5-6 times a week) compared to in center (3 times a week) dialysis [61]. Studies suggest improvement of SD in men after renal transplantation. However, half of the men with renal transplant still have symptoms of SD [62]. Recipients of renal allograft with ED are candidates for treatment with PDE5i [63]
Conclusion
Sexual dysfunction and endocrine hormonal abnormalities are common in patient on renal replacement therapy and are associated with lower Qol. In men, low testosterone levels are associated with increased cardiovascular mortality. Phosphodiesterase 5 inhibitors are effective treatment for erective dysfunction in men and should be considered as first line therapy after optimizing effectiveness and adequacy of renal replacement therapy. Testosterone replacement alone may not produce adequate effects. The issue of SD is more complicated in women and although highly prevalent, therapeutic options are limited. Renal transplant offers the best treatment options for women of child bearing age who have sexual dysfunction.
References
- Basok EK, Atsu N, Rifaioglu MM, Kantarci G, Yildirim A, Tokuc R. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int Urol Nephrol. 2009; 41: 473-481.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999; 281: 537-544.
- Giraldi A, Kristensen E. Sexual dysfunction in women with diabetes mellitus. J Sex Res. 2010; 47: 199-211.
- Anantharaman P, Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis. 2007; 14: 119-125.
- Masters WH, Johnson VE. Human sexual response. Little, Brown and Company, Boston 1966
- Access Medicine. McGraw-Hill Companies. Endocrine physiology 3rd edition. Chapter 8. Male reproductive system
- Access Medicine. McGraw-Hill Companies. Endocrine physiology 3rd edition. Chapter 9. Female reproductive system
- Guvel S, Pourbagher MA, Torun D, Egilmez T, Pourbagher A, Ozkardes H. Calcification of the epididymis and the tunica albuginea of the corpora cavernosa in patients on maintenance hemodialysis. J Androl. 2004; 25: 752-756.
- Rosas SE, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, Grossman E. Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int. 2001; 59: 2259-2266.
- Arslan D, Aslan G, Sifil A, Cavdar C, Celebi I, Gamsari T, et al. Sexual dysfunction in male patients on hemodialysis: assessment with the International Index of Erectile Function (IIEF). Int J Impot Res. 2002; 14: 539-542.
- The Kidney. Brenner and Rector. 2008. 8th edition, Volume 2. Chapter 50. Endocrine aspect of kidney diseases
- Nassir A1. Erectile dysfunction risk factors for patients entering dialysis programme. Andrologia. 2010; 42: 41-47.
- Peng YS, Chiang CK, Kao TW, Hung KY, Lu CS, Chiang SS, et al. Sexual dysfunction in female hemodialysis patients: a multicenter study. Kidney Int. 2005; 68: 760-765.
- Vecchio M, Navaneethan SD, Johnson DW, Lucisano G, Graziano G, Querques M, et al. Treatment options for sexual dysfunction in patients with chronic kidney disease: a systematic review of randomized controlled trials. Clin J Am Soc Nephrol. 2010; 5: 985-995.
- Hladunewich MA, Hou S, Odutayo A, Cornelis T, Pierratos A, Goldstein M, et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol. 2014; 25: 1103-1109.
- Kaufman JM, Hatzichristou DG, Mulhall JP, Fitch WP, Goldstein I. Impotence and chronic renal failure: a study of the hemodynamic pathophysiology. J Urol. 1994; 151: 612-618.
- Pu XY, Wang XH, Gao WC, Yang ZH, Li SL, Wang HP, et al. Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. J Sex Med. 2008; 5: 1345-1354.
- Kim N, Vardi Y, Padma-Nathan H, Daley J, Goldstein I, Saenz de Tejada I. Oxygen tension regulates the nitric oxide pathway. Physiological role in penile erection. J Clin Invest. 1993; 91: 437-442.
- Johnson S, Dwyer A. Patient perceived barriers to treatment of depression and anxiety in hemodialysis patients. Clin Nephrol. 2008; 69: 201-206.
- Seibel I, Poli De Figueiredo CE, Telöken C, Moraes JF. Efficacy of oral sildenafil in hemodialysis patients with erectile dysfunction. J Am Soc Nephrol. 2002; 13: 2770-2775.
- Mahon A, Sidhu PS, Muir G, Macdougall IC. The efficacy of sildenafil for the treatment of erectile dysfunction in male peritoneal dialysis patients. Am J Kidney Dis. 2005; 45: 381-387.
- Turk S, Solak Y, Kan S, Atalay H, Kilinc M, Agca E, et al. Effects of sildenafil and vardenafil on erectile dysfunction and health-related quality of life in haemodialysis patients: a prospective randomized crossover study. Nephrol Dial Transplant. 2010; 25: 3729-3733.
- Lasaponara F, Sedigh O, Pasquale G, Bosio A, Rolle L, Ceruti C, et al. Phosphodiesterase type 5 inhibitor treatment for erectile dysfunction in patients with end-stage renal disease receiving dialysis or after renal transplantation. J Sex Med. 2013; 10: 2798-814.
- Grossman EB, Swan SK, Muirhead GJ, Gaffney M, Chung M, DeRiesthal H, et al. The pharmacokinetics and hemodynamics of sildenafil citrate in male hemodialysis patients. Kidney Int. 2004; 66: 367-374.
- Muirhead GJ, Rance DJ, Walker DK, Wastall P. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil. Br J Clin Pharmacol. 2002; 53 Suppl 1: 13S-20S.
- Muirhead GJ, Wilner K, Colburn W, Haug-Pihale G, Rouviex B. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. Br J Clin Pharmacol. 2002; 53 Suppl 1: 21S-30S.
- LEVITRA® (vardenafil). Full Prescribing Information. Bayer Pharmaceuticals Corporation, West Haven, CT. 2008.
- Jacques J, Abrairs C. Quality-of-life issues in hemodialysis: Case studies on the use of sildenafil for erectile dysfunction. Dial Transplant 1999; 28:519â“24.
- Chen J, Mabjeesh NJ, Greenstein A, Nadu A, Matzkin H. Clinical efficacy of sildenafil in patients on chronic dialysis. J Urol. 2001; 165: 819-821.
- Punzo G, Maggi S, Ponzio R, Costarella M, Gentile V. [Use of sildenafil in the chronic uremic patient]. Minerva Urol Nefrol. 2001; 53: 39-43.
- Rosas SE, Wasserstein A, Kobrin S, Feldman HI. Preliminary observations of sildenafil treatment for erectile dysfunction in dialysis patients. Am J Kidney Dis. 2001; 37: 134-137.
- Türk S, Karalezli G, Tonbul HZ, Yildiz M, Altintepe L, Yildiz A, et al. Erectile dysfunction and the effects of sildenafil treatment in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2001; 16: 1818-1822.
- YeniçerioGlu Y, Kefi A, Aslan G, Cavdar C, Esen AA, Camsari T, et al. Efficacy and safety of sildenafil for treating erectile dysfunction in patients on dialysis. BJU Int. 2002; 90: 442-445.
- Hyodo T, Ishida H, Masui N, Taira T, Yamamoto S, Oka M, et al. Kidney disease quality of life of Japanese dialysis patients who desire administration of sildenafil and the treatment of erectile dysfunction using sildenafil. Ther Apher Dial. 2004; 8: 340-360.
- Sahin Y, Aygün C, PeÅkircioÄlu CL, Kut A, Tekin MI, Ozdemir FN, OzkardeÅ H. Efficacy and safety of sildenafil citrate in hemodialysis patients. Transplant Proc. 2004; 36: 56-58.
- Dachille G, Pagliarulo V, Ludovico GM, Ralph D, Pagliarulo A. Sexual dysfunction in patients under dialytic treatment. Minerva Urol Nefrol. 2006; 58: 195-200.
- Tas A, Ersoy A, Ersoy C, Gullulu M, Yurtkuran M. Efficacy of sildenafil in male dialysis patients with erectile dysfunction unresponsive to erythropoietin and/or testosterone treatments. Int J Impot Res. 2006; 18: 61-68.
- Ghafari A, Farshid B, Afshari AT, Sepehrvand N, Rikhtegar E, Ghasemi K, et al. Sildenafil citrate can improve erectile dysfunction among chronic hemodialysis patients. Indian J Nephrol. 2010; 20: 142-145.
- Khurana KK, Navaneethan SD, Arrigain S, Schold JD, Nally JV Jr, Shoskes DA. Serum testosterone levels and mortality in men with CKD stages 3-4. Am J Kidney Dis. 2014; 64: 367-374.
- Bello AK, Stenvinkel P, Lin M, Hemmelgarn B, Thadhani R, Klarenbach S, Chan C. Serum testosterone levels and clinical outcomes in male hemodialysis patients. Am J Kidney Dis. 2014; 63: 268-275.
- Carrero JJ, Qureshi AR, Nakashima A, Arver S, Parini P, Lindholm B, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant. 2011; 26: 184-190.
- Lawrence IG, Price DE, Howlett TA, Harris KP, Feehally J, Walls J. Correcting impotence in the male dialysis patient: experience with testosterone replacement and vacuum tumescence therapy. Am J Kidney Dis. 1998; 31: 313-319.
- Cangüven O, Aykose G, Albayrak S, Goktas C, Horuz R, Yencilek F. Efficacy of testosterone gel in the treatment of erectile dysfunction in hypogonadal hemodialysis patients: a pilot study. Int J Impot Res. 2010; 22: 140-145.
- Chatterjee R, Wood S, McGarrigle HH, Lees WR, Ralph DJ, Neild GH. A novel therapy with testosterone and sildenafil for erectile dysfunction in patients on renal dialysis or after renal transplantation. J Fam Plann Reprod Health Care. 2004; 30: 88-90.
- Lei KY, Abbasi A, Prasad AS. Function of pituitary-gonadal axis in zinc-deficient rats. Am J Physiol. 1976; 230: 1730-1732.
- Antoniou LD, Shalhoub RJ, Sudhakar T, Smith JC Jr. Reversal of uraemic impotence by zinc. Lancet. 1977; 2: 895-898.
- Jalali GR, Roozbeh J, Mohammadzadeh A, Sharifian M, Sagheb MM, Hamidian Jahromi A, Shabani S. Impact of oral zinc therapy on the level of sex hormones in male patients on hemodialysis. Ren Fail. 2010; 32: 417-419.
- Mahajan SK, Abbasi AA, Prasad AS, Rabbani P, Briggs WA, McDonald FD. Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Ann Intern Med. 1982; 97: 357-361.
- Brook AC, Johnston DG, Ward MK, Watson MJ, Cook DB, Kerr DN. Absence of a therapeutic effect of zinc in the sexual dysfunction of haemodialysed patients. Lancet. 1980; 2: 618-620.
- Strippoli GF; Collaborative Depression and Sexual Dysfunction (CDS) in Hemodialysis Working Group, Vecchio M, Palmer S, De Berardis G, Craig J, et al.Sexual dysfunction in women with ESRD requiring hemodialysis. Clin J Am SocNephrol. 2012 ;7: 974-81.
- Mor MK, Sevick MA, Shields AM, Green JA, Palevsky PM, Arnold RM, et al. Sexual function, activity, and satisfaction among women receiving maintenance hemodialysis. Clin J Am Soc Nephrol. 2014; 9: 128-134.
- Lopes GB, Matos CM, Leite EB, Martins MT, Martins MS, Silva LF, et al. Depression as a potential explanation for gender differences in health-related quality of life among patients on maintenance hemodialysis. Nephron ClinPract. 2010; 115: 35-40.
- Filocamo MT, Zanazzi M, Li Marzi V, Lombardi G, Del Popolo G, Mancini G, et al. Sexual dysfunction in women during dialysis and after renal transplantation. J Sex Med. 2009; 6: 3125-3131.
- Lawrence IG, Price DE, Howlett TA, Harris KP, Feehally J, Walls J. Erythropoietin and sexual dysfunction. Nephrol Dial Transplant. 1997; 12: 741-747.
- Wu SC, Lin SL, Jeng FR. Influence of erythropoietin treatment on gonadotropic hormone levels and sexual function in male uremic patients. Scand J Urol Nephrol. 2001; 35: 136-140.
- Yeksan M, Tamer N, Cirit M, Türk S, Akhan G, Akkus I, et al. Effect of recombinant human erythropoietin (r-HuEPO) therapy on plasma FT3, FT4, TSH, FSH, LH, free testosterone and prolactin levels in hemodialysis patients. Int J Artif Organs. 1992; 15: 585-589.
- Peng YS, Chiang CK, Hung KY, Chiang SS, Lu CS, Yang CS, et al. The association of higher depressive symptoms and sexual dysfunction in male haemodialysis patients. Nephrol Dial Transplant. 2007; 22: 857-861.
- Weisbord SD, Mor MK, Green JA, Sevick MA, Shields AM, Zhao X, et al. Comparison of symptom management strategies for pain, erectile dysfunction, and depression in patients receiving chronic hemodialysis: a cluster randomized effectiveness trial. Clin J Am SocNephrol. 2013; 8:90-9.
- Chou FF, Lee CH, Shu K, Yu TJ, Hsu KT, Sheen-Chen SM. Improvement of sexual function in male patients after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2001; 193: 486-492.
- Blumberg A, Wildbolz A, Descoeudres C, Hennes U, Dambacher MA, Fischer JA, Weidmann P. Influence of ,25 dihydroxycholecalciferol on sexual dysfunction and related endocrine parameters in patiens on maintenance hemodialysis. Clin Nephrol. 1980; 13: 208-214.
- Bass A, Ahmed SB, Klarenbach S, Culleton B, Hemmelgarn BR, Manns B. The impact of nocturnal hemodialysis on sexual function. BMC Nephrol. 2012; 13: 67.
- Malavaud B, Rostaing L, Rischmann P, Sarramon JP, Durand D. High prevalence of erectile dysfunction after renal transplantation. Transplantation. 2000; 69: 2121-2124.
- Sharma RK, Prasad N, Gupta A, Kapoor R. Treatment of erectile dysfunction with sildenafil citrate in renal allograft recipients: a randomized, double-blind, placebo-controlled, crossover trial. Am J Kidney Dis. 2006; 48:128-33.
Citation: Koc ZP, Balci TA and Dogukan A. Single Photon Emission Tomography (Spect) Imaging is Essential to Show Hernia of Peritoneum in Peritoneal Scintigraphy in Peritoneal Dialysis Patients. Austin J Nephrol Hypertens. 2015;2(1): 1030.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964