
Case Report
Austin J Nephrol Hypertens. 2015;2(1): 1032.
Simultaneous Renal Transplantation and Splenectomy: Double Strike?
William EA1,2*, Morsy AA1, Iskander IR1 and Barsoum RS1
1The Cairo Kidney Center, Cairo University, Egypt
2Department of Internal Medicine, National Research Center, Egypt
*Corresponding author: William EA, The Cairo Kidney Center, 3 Hussein El-Memar Street, Cairo, PO Box 91 Bab-El-Louk, Cairo 11513, Egypt
Received: November 28, 2014; Accepted: January 27, 2015; Published: January 29, 2015
Abstract
We present a 59 year-old female suffering from idiopathic thrombocytopenic purpura and end stage renal disease due to mesangiocapillary (Type III) glomerulonephritis, who received a kidney transplant from a living unrelated male donor. Aiming for a definite and durable management for idiopathic thrombocytopenic purpura and because of calcified pelvic vessels, she had simultaneous splenectomy and left orthotopic renal transplantation after only pulse-steroid induction. Post-transplant immunosuppression comprised tacrolimus, mycophenolic acid and prednisolone. Graft functioned well, apart from transient rise of serum creatinine after few days due to tacrolimus induced thrombotic microangiopathy. The offending drug was withdrawn under cover of 2 doses of basiliximab. The patient achieved normal kidney function thereafter and cyclosporine was introduced instead of tacrolimus. Unfortunately, she experienced a chain of infections caused by different bacterial and fungal infections, starting with superficial wound infection and followed by urinary tract infection and septicemia, which turned out to be superimposed on cytomegaloviral (CMV) activation. Lowering immunosuppressive drug doses, antiviral treatment and culture-based antibiotics failed to control these infections, leading to the patient’s death with a functioning graft, 1 month after transplantation.
Conclusion: despite surgically feasible, simultaneous splenectomy and renal transplantation may carry the risk of over immunosuppression and potentially fatal infection.
Keywords: Idiopathic thrombocytopenic purpura; Renal transplantation; Splenectomy; Cytomegalovirus (CMV); Mesangiocapillary glomerulonephritis
Introduction
Infection remains the second most common cause of death after cardiovascular causes in kidney transplant recipients. It accounted for 18.8 % of patient’s death in the 2013 USRDS annual data report [1], and 15 % In the ERA-EDTA registry [2]. Nevertheless, certain clinical situations may impose the creation of an environment of over-immunosuppression as in this case.
Case Report
Our patient was a 59 year-old female with long standing hypertension (35 years), Type 2 diabetes (8 years), and multiple autoimmune disorders including hemolytic anemia (12 years), interstitial lung disease (11 years) and thrombocytopenia (1 year). Serological tests for SLE were repeatedly negative. She had a single seizure at 16 years ago with negative Magnetic Resonance Imaging, and deep vein thrombosis of the right lower limb at age 36 years. Impairment of renal function was detected at age 49, with steady progression to end-stage kidney disease (ESKD) in 10 years.
Her chronic kidney disease (CKD) was evaluated when she was 54 years old. She was clinically well, with adequately controlled blood pressure, and no abnormal physical signs apart from a faint hemic cardiac murmur and a palpable spleen 3cm below the costal margin. The ocular fundi showed grade II hypertensive changes, without diabetic manifestations. Midstream urine showed bland sediment with a urinary protein/creatinine ratio 11.5 mg/mg. Serum creatinine was 265 μmol/l, with eGFR (MDRD-4) of 16.3 ml/min/sqm. Serum ANA, anti-DNA, Fractional Extractable Nuclear Antigens and anticardiolipin antibodies were negative. Serum levels of complement C3, C4and CH50 were normal. By ultrasonographic examination, the kidneys were of normal size, yet increased echogenicity. Renal biopsy showed mesangiocapillary (Type III) changes and segmental sclerosis with negative immunofluorescence for IgG, IgM, IgA and complement. She was thus kept on standard conservative management.
She was re-assessed 2 years later, when serum creatinine had reached 309 μmol/l. Her anti-nuclear, anticardiolipin, and anti RNP- 70 antibodies were detected in her serum. Repeat renal biopsy showed the same mesangiocapillary changes, yet with advanced focal and global sclerosis. She received a therapeutic trial with prednisolone and azathioprine, without benefit. She eventually passed to ESKD and started regular hemodialysis.
She was living alone with her daughter and needing her assistance for transportation which interfered with her job. She became depressed and frightened from dialysis and opted to receive a transplant. Preoperative transthoracic echocardiogram showed fair systolic function (ejection fraction 55%) and old rheumatic valve disease in the form of moderate mitral regurgitation and mild mitral stenosis. Neither Rt. nor Lt. Cardiac catheterization were done. Spirometry showed moderate restrictive lung disease, however, chest x-ray and arterial blood gasses were normal. CT angiography showed extensive calcification of the abdominal aorta and iliac arteries. Her platelet count was 62,000/c.mm, with absent megakaryocytic budding in the bone marrow.
We designed a plan for proceeding to transplantation in three steps: a) temporarily correcting the platelet count for safe surgery; b) splenectomy for long-term management of her thrombocytopenia; c) simultaneous orthotopic renal transplantation using the splenic artery to avoid the calcified other abdominal vessels, which wouldn’t have been possible in a staged surgery.
She was vaccinated against meningococcus, H.influenza, and pneumococcus 1 month before scheduled surgery.
An attempt to correct the platelet count by prednisolone was partially successful yet un-sustained. So, she was treated with Intravenous Immunoglobulin, in a total dose of 500mg/Kg spread over 4 days, which raised the platelet count over 100,000/c.mm that was sustained throughout the surgery and early post-operative period.
The donor was a healthy live, 25-year old male with matching ABO, cde antigens (A1+), 3/6 mismatching HLA antigens (A3, A30, B18) and a negative lymphocytotoxicity cross match. Both patient and donor sera were negative for HBV, HCV and HIV markers, yet with positive anti-CMV IgG antibodies.
Only steroids were used for induction, given as methyl prednisolone in 3 doses: 250 mg at -12h, 500 mg at 0h and 250 mg at +12h.
Two surgical teams performed the operation on 31st March 2013. While Team A was performing the recipient splenectomy and left nephrectomy, Team B was doing the donor nephrectomy. Both procedures were uneventful.
The recipient’s left renal artery turned out to be of a wider caliber and more accessible for anastomosis; so it was used end-to-end for the graft anastomosis, instead of the planned splenic artery. The graft’s vein was anastomosed to the native left renal vein. The graft’s ureter was sutured to the native left ureter, and a double J catheter was left across the new ureteral anastomosis. The total ischemia time was 45 minutes.
Maintenance immunosuppression comprised Tacrolimus (3 mg) and Mycophenolic acid (360mg) twice daily and prednisolone in daily decremented doses from 100 mg on day +1 to 20 mg on day +7. Ceftazidime was used for chemoprophylaxis in a daily i.v. dose of 1gm.
Diuresis started 6 hours postoperatively and accelerated over the following days, with drop of serum creatinine from 375 μmol/l to 176 μmol/l by day +4. However, it rose to 232 μmol/l on day +5 along with reduction of urine volume. Simultaneously, the tacrolimus trough blood level was 17.4 ng/ml. The dose was reduced to 2mg twice daily, yet serum creatinine continued to rise. By day +7, a graft biopsy was obtained and showed a thrombotic microangiopathy (Figure 1a,1b).
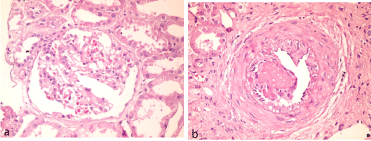
Figure 1: Graft biopsy on day 7. a) Ischemic glomerulus with multiple capillary thrombi and surrounding tubular atrophy; b) Small artery showing endothelial cell
swelling and proliferation with a large thrombus. (H & E stain, Original magnification x400).
Tacrolimus was withdrawn under cover of 2 doses of Basiliximab, 20 mg each, administered on days +8 and +12. This resulted in rapid improvement of graft function, with serum creatinine declining to 88.4 ìmol/l in a few days.
Unfortunately, the wound got superficially infected; culture showed a candidal growth and intravenous fluconazole was added. All tubes and catheters were removed, including the central venous line, urinary catheter and wound drain.
Although the signs of infection improved, she complained of shortness of breath, with atrial fibrillation and pulmonary hypertension (Pulmonary artery systolic pressure 75mm Hg) by echocardiographic examination. This was treated with diuretics, nitroglycerine and amiodarone, silendafil. Although D-dimer was not detected, enoxaparin was added with good response. Cyclosporine was cautiously introduced on day +14 in a daily dose of 100 mg and she was discharged in good shape, normal leucocytic count (9500/c. mm), and normal graft function (serum creatinine 61 μmol/l).
A few days after discharge, she became febrile (up to 39°C), progressively catabolic, with remarkable asthenia and peripheral edema. The wound started to discharge again and she developed a left pleural effusion. The leucocytic count showed a left shift up to 24% with a rising total count, serum creatinine showed a mild elevation (132 ìmol/l) and serum albumin a decline (26 gm/l). Intraabdominal collection was excluded by ultrasonic examination. A diagnostic sample from the left pleural effusion showed the features of a transudate with negative culture.
Wound, urine and blood cultures showed multiple organisms including E.Coli, pseudomonas and candida, for which immunosuppression was reduced to minimal doses, and she received appropriate antibiotics intravenously. The double J catheter was removed when backpressure manifestations were detected by ultrasonographic follow-up.
The full-blown picture of CMV syndrome eventually evolved, with hyperbiliribinemia (27.3 μmol/l) and elevated hepatic transaminases (double normal), uncontrolled sepsis, shock and eventual death despite antiviral treatment, 1 month after transplantation.
Discussion
The decision to proceed to simultaneous splenectomy and kidney transplantation in this case was inevitable. Her thrombocytopenia was a part of a train of autoimmune disorders and her CKD, attributed to Type III mesangiocapillary glomerulonephritis, was a typical reflection of her autoimmune disorder.
The low platelet count would have been a constant threat if she were to continue on regular hemodialysis, hence the decision to treat it despite a platelet count above 30,000/c.mm [3]. Attempts to raise the platelet count by corticosteroids were only partially effective and short-lived. Therefore, the decision was to consider simultaneous splenectomy and renal transplantation.
This complex surgery has been practiced since the early days of transplantation with confusing results [4]. It is currently reserved for pre-sensitized recipients [5] and ABO incompatible transplants [6]. It was also used in occasional patients where calcifications of the aorta and iliac arteries did not permit positioning of the graft in the iliac fossa as usual [7]. Overall experience suggests augmented immunosuppression, yet at the expense of additional risk of surgical complications [8] and infection [9], particularly with pneumococcus, meningococcus and H. influenza [10].
Our patient was adequately prepared by preoperative vaccination [11] and her platelet count was raised to a safe level by intravenous immunoglobulin [12].
Thrombotic microangiopathy may have been facilitated by the pre-existing anticardiolipin antibodies [13]. Nevertheless, tacrolimus withdrawal under cover of Basiliximab, restored normal graft function, and permitted late introduction of Cyclosporine A, which is in-line with other experiences [14].
Anticardiolipin antibodies may have been also responsible for the formation of undetected pelvic or lower limb thrombi that showered into the pulmonary circulation [15]. This diagnosis was made despite a negative D-dimer test, which concurs with other experiences [16].
Splenectomy must be blamed for the infectious complications. Beside these bacterial and fungal scenarios, there was an ongoing sinister reactivation of CMV infection that was further depressing the patient’s immune response, in addition to its own harm. CMV activation is often associated with immunosuppression for transplantation, particularly the bone marrow [17], as well as in solid organs transplantation [18]. The role of splenectomy in activating a dormant CMV infection has been shown in humans[19].
Conclusion
While simultaneous splenectomy and renal transplantation was technically successful, removing the spleen seems to have been responsible for over-immunosuppression leading to a fatal sepsis and probably CMV activation.
References
- 2013 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. American Journal of Kidney Diseases. 2014; 63: Supplement e1-e478.
- Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001; 16: 1545-1549.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117: 4190-4207.
- Sutherland DE, Fryd DS, So SK, Bentley FR, Ascher NL, Simmons RL, Najarian JS. Long-term effect of splenectomy versus no splenectomy in renal transplant patients. Reanalysis of a randomized prospective study. Transplantation. 1984; 38: 619-624.
- Tzvetanov I, Spaggiari M, Jeon H, Roca RG, Bhati C, Oberholzer J, Benedetti E. The role of splenectomy in the setting of refractory humoral rejection after kidney transplantation. Transplant Proc. 2012; 44: 1254-1258.
- Hayashi T, Tahara H, Hara Y, Ishii T, Uemura H. Experience With Laparoscopic Splenectomy for ABO-Incompatible Living Renal Transplantation Without Plasmapheresis. Transplant Proc. 2006; 38: 1985-6.
- Paduch DA, Barry JM, Arsanjani A, Lemmers MJ. Indication, surgical technique and outcome of orthotopic renal transplantation. J Urol. 2001; 166: 1647-1650.
- Pate JW, Peters TG, Andrews CR. Postsplenectomy complications. Am Surg. 1985; 51: 437-441.
- Peters TG, Williams JW, Harmon HC, Britt LG. Splenectomy and death in renal transplant patients. Arch Surg. 1983; 118: 795-799.
- Moxon ER, Goldthorn JF, Schwartz AD. Haemophilus influenzae b infection in rats: effect of splenectomy on bloodstream and meningeal invasion after intravenous and intranasal inoculations. Infect Immun. 1980; 27: 872-875.
- Spelman D, Buttery J, Daley A, Isaacs D, Jennens I, Kakakios A, Lawrence R. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J. 2008; 38: 349-356.
- Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005; 106: 2244-2251.
- Kon Y, Atsumi T, Hagiwara H, Furusaki A, Kataoka H, Horita T, et al. Thrombotic microangiopathy in patients with phosphatidylserine dependent antiprothrombin antibodies and antiphospholipid syndrome. Clin Exp Rheumatol. 2008; 26: 129-32.
- Lin CC, King KL, Chao YW, Yang AH, Chang CF, Yang WC. Tacrolimus-associated hemolytic uremic syndrome: a case analysis. J Nephrol. 2003; 16: 580-585.
- Stone JH, Amend WJ, Criswell LA. Antiphospholipid antibody syndrome in renal transplantation: occurrence of clinical events in 96 consecutive patients with systemic lupus erythematosus. Am J Kidney Dis. 1999; 34: 1040-7.
- Ireland B1. Negative ELISA D-dimer assay can miss pulmonary embolism. J Fam Pract. 2003; 52: 99, 103.
- Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011; 25: 151-169.
- Razonable RR, Humar A. AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013; 4: 93-106.
- Han XY, Lin P, Amin HM, Ferrajoli A. Postsplenectomy cytomegaloviral mononucleosis: marked lymphocytosis, TCRgamma gene rearrangements, and impaired IgM response. Am J Clin Pathol. 2005; 123: 612-617.
Citation: William EA, Morsy AA, Iskander IR and Barsoum RS. Simultaneous Renal Transplantation and Splenectomy: Double Strike?. Austin J Nephrol Hypertens. 2015;2(1): 1032.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964