
Case Report
Austin J Nephrol Hypertens. 2015;2(2): 1034.
Quadruple Nephropathy in a Liver Transplant Patient
Dominic Rossini, Youngki Kim and Marc Weber*
Division of Renal Diseases and Hypertension, University of Minnesota, USA
*Corresponding author: Marc Weber, Division of Renal Diseases and Hypertension, University of Minnesota, 717 Delaware Street SE, Mail Code 1932, Minneapolis, MN 55414
Received: November 26, 2014; Accepted: February 02, 2015; Published: February 04, 2015
Abstract
Hepatitis C Virus (HCV) is associated with multiple renal diseases, namely cryoglobulinemic Membranoproliferative Glomerulonephritis (MPGN), IgA nephropathy, and diabetic nephropathy. The pattern of renal injury in patients with HCV-associated cryoglobulinemia is MPGN in nearly 80% of cases. The pathogenesis linking IgA nephropathy with HCV and cirrhosis remains unclear. HCV is associated with an increased risk of type 2 diabetes mellitus. Cirrhotic patients are at risk for Acute Tubular Necrosis (ATN) due to a tenuous hemodynamic state. We describe a case of quadruple nephropathy in a patient with HCV and diabetes mellitus who develops MPGN, IgA nephropathy, diabetic nephropathy and ATN.
Keywords: Hepatitis C; Quadruple Nephropathy
Background
Hepatitis C Virus (HCV) infection is a leading cause of cirrhosis, but is also associated with a number of renal diseases, namely cryoglobulinemic Membranoproliferative Glomerulonephritis (MPGN), IgA nephropathy (IgAN), and Diabetic Nephropathy (DN) [1-3]. HCV is the most common blood-borne infection in the United States, affecting nearly 3.2 million Americans. HCV is associated with an increased risk for insulin resistance and Diabetes Mellitus (DM), which is the most common cause of ESKD in the United States [4]. While mixed cryoglobulinemia has a firm association with HCV and a MPGN pattern of renal injury, the pathogenesis behind HCV related IgAN remains less well understood. We describe a case of quadruple nephropathy in a patient with HCV infection and DM who develops MPGN, IgAN, DN and ATN.
Case Report
A 55 year old male with a four year history of type 2 DM and recurrent compensated chronic hepatitis C (+ serum HCV RNA PCR and histological diagnosis of recurrent HCV cirrhosis with mild activity and advanced bridging fibrosis) 4 years after liver transplantation presented to our clinic with a creatinine of 1.45mg/ dl (baseline 0.8 mg/dl). His immunosuppression regimen over the past year prior to presentation included tacrolimus (levels between 4-9) and prednisone. He was not exposed to obvious nephrotoxins. He had microscopic hematuria and the urine protein to creatinine ratio was 6g/g. Serologic work-up revealed low C3 and C4, positive RF and trace cryoglobulins. He did not have myeloma, lupus or other autoimmune disease.
His kidney biopsy was significant for features of MPGN with diffuse glomerular basement membrane thickening, irregularity, and duplication in setting of mildly enlarged glomeruli with increased cellularity as well as endocapillary proliferation. There was also evidence of mild ATN by light microscopy. Immunofluorescence studies showed diffuse granular IgM, IgA, C3, C1q, and kappa light chain staining along the subendothelial area of glomerular capillary walls in a peripheral lobular distribution. There was also diffuse global granular mesangial staining for IgA, C3, and lambda light chain. There was thick linear staining of GBM and TBM for IgG and albumin. Albumin also showed global mesangial staining. Congo Red staining was negative. Electron microscopy showed foot process effacement and subendothelial electron-dense immune complex deposition with similar mesangial deposits.
Unfortunately, in the weeks following the kidney biopsy the patient was admitted to the hospital with community acquired pneumonia, developed sepsis with multi-organ failure and died.
Discussion
This patient had evidence of four renal pathological processes. His rise in creatinine was likely due to ATN, the etiology of which remains unclear. He did not have a recent history of elevated tacrolimus levels, documented hypotensive episodes, or known exposure to nephrotoxins. He had clear pathological hallmarks of MPGN (lobular glomerular appearance and “tram-tracking” by light microscopy (Figure 1A and 1B)) with immunofluorescence-staining suggestive of mixed cryoglobulinemia (peripheral subendothelial staining pattern for IgG (Figure 1C), C3 (Figure 1D), IgM (Figure 1E) and kappa light chain (Figure 1F)).
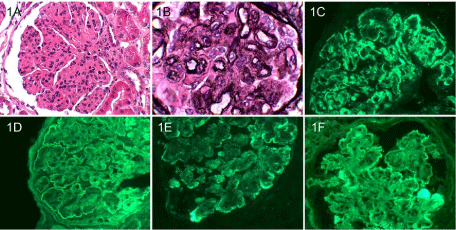
Figure 1: Light microscopy evidence of lobular appearing glomeruli and “tramtracking”
and immunofluorescence evidence of a peripheral subendothelial
staining pattern for IgG (Figure 1C), C3 (Figure 1D), IgM (Figure 1E) and
kappa light chain(Figure 1F).
There was also immunofluorescence staining consistent with IgA nephropathy (mesangial IgA (Figure 2A) and C3 (Figure 2B)) as well as diabetic nephropathy (linear glomerular basement for albumin (Figure 3A) and tubular basement membranestaining for IgG (Figure 3B). Electron microscopy supported the diagnoses of IgA nephropathy with mesangial deposits (Figure 4A) and cryoglobulinemic glomerulonephritis with subendothelial immune complex deposits (Figure 4B).
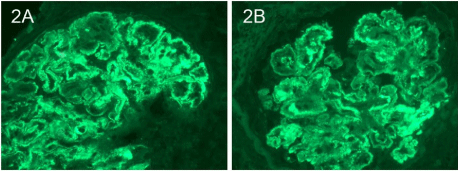
Figure 1: Immunofluorescence staining of mesangial IgA (Figure 2A) and
C3 (Figure 2B).
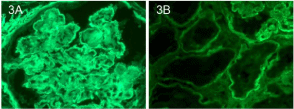
Figure 3: Linear glomerular basement staining for albumin (Figure 3A) and
tubular basement membrane staining for IgG (Figure 3B).
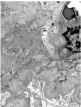
Figure 4A: Electron microscopy evidence of mesangial deposits.

Figure 4b: Electron microscopy evidence subendothelial immune complex
deposits.
The prevalence of cryoglobulins in patients with HCV infection ranges from 19% to 41.5% [5]. HCV is the cause of 80%-90% of mixed cryoglobulinemia and is associated with type II and type III mixed cryoglobulinemia. Mixed cryoglobulinemia represents a form of immune complex vasculitis caused by an increase in B-cell production of IgM with rheumatoid factor activity that can accumulate in glomeruli.
The pathological pattern of renal injury in patients with HCVassociated cryoglobulinemia is MPGN in nearly 80% of cases and is the most frequently associated HCV renal disease [6,7]. Eosinophilic thrombi containing cyroglobulin are sometimes present in the glomerular capillaries and are associated with glomerular necrosis; although this was not found in the patient described in this case report.
The presence of mesangial IgA deposits in patients with cirrhosis was first described in the 1970’s in autopsy series reports [8]. Mesangial granular IgA deposits were found in 58 of 100 cirrhotic patients at autopsy. Several other reports suggest an association between alcoholic cirrhosis and IgA deposition [9]. The precise pathogenesis behind such associations remains unclear. HCV-associated IgAN is thought to be a result of immune complex deposition induced by both the HCV antigens and impaired hepatic clearance of circulating IgA [10]. Abnormal IgA glycosylation has been implicated in mesangial deposition of IgA and resultant IgAN [11]. The plasma level of IgA is determined by the relative rates of IgA synthesis and IgA removal by hepatocytes. Cirrhotic patients who lack the normal number of functioning hepatocytes may be unable to catabolize IgA molecules at a normal rate, resulting in elevated plasma levels of IgA. However, elevated serum IgA alone does not necessarily result in mesangial deposition or the development of IgA nephropathy. Patients with IgA myeloma often have high IgA serum levels and IgA nephropathy is uncommon in these patients. The normal catabolic pathway for circulating IgA1 molecules is via the asialoglycoprotein receptor (ASGP-R) on hepatocytes which recognizes terminal galactose residues [12]. The ability of the ASGP-R to clear under-galactosylated IgA1 in patients with IgA nephropathy has not been explained [13] but it is possible that galactose-deficient IgA1 is not recognized by ASPG-R and is able to escape the normal IgA1 catabolic pathway. Furthermore, circulating IgA1-IgG immune complexes in patientswith IgAN may be too large (~800 kD) to allow entry into the hepatic space of Disuse and therefore lack interaction with ASPG-R, resulting in reduced plasma removal. This could explain why hepatic clearance of IgA1 is decreased in patients with IgA nephropathy [14] but does not completely explain the association of HCV with IgA nephropathy.
Chronic HCV is associated with hepatic steatosis, Insulin Resistance (IR) and increased risk of type 2 Diabetes Mellitus (DM2) [15]. Successful treatment of HCV infection can improve insulin resistance and hepatic steatosis however; these problems often recur as HCV infection returns [15]. This suggests an intimate relationship between HCV and risk of DM2.
Mehta et al performed a prospective analysis to examine if patients who acquired DM2 were more likely to have had antecedent HCV infection. They found an increased incidence of DM2 in patients with recognized diabetes risk factors and HCV infection when compared to patients without HCV infection but with similar diabetes risk factors [16]. More interesting are the results of a study by Kim et al [17] who analyzed renal biopsy specimens from 81 OLT recipients (the most common reason for OLT was HCV in 51% of patients) who all underwent renal biopsy for impaired renal function at a mean of 4.95 years after transplant. Among patients without a clinical diagnosis of DM2 and normal HgbA1C, biopsies showed glomerular basement membrane thickening in 39% and diffuse nodular glomerulosclerosis in 16%. These results suggest pathologic findings of diabetic nephropathy in patients without clinical DM2.
To our knowledge this is the first case report describing four distinct renal pathologic entities including ATN, IgAN, cryoglobulinemic glomerulonephritis with MPGN, and DN. This case report highlights the importance of entertaining a broad differential diagnosis in patients with hepatitis C and cirrhosis. A better understanding of how HCV infection precipitates development of common renal and systemic diseases may provide unique insights into pathogenesis and treatment of these disease states.
References
- Sumida K1, Ubara Y, Hoshino J, Suwabe T, Nakanishi S, Hiramatsu R, Hasegawa E . Hepatitis C virus-related kidney disease: various histological patterns. See comment in PubMed Commons below Clin Nephrol. 2010; 74: 446-456.
- Kawaguchi K, Koike M . Glomerular lesions associated with liver cirrhosis: an immunohistochemical and clinicopathologic analysis. See comment in PubMed Commons below Hum Pathol. 1986; 17: 1137-1143.
- Fukuda Y . Renal glomerular changes associated with liver cirrhosis. See comment in PubMed Commons below Acta Pathol Jpn. 1982; 32: 561-574.
- White DL1, Ratziu V, El-Serag HB . Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. See comment in PubMed Commons below J Hepatol. 2008; 49: 831-844.
- Agnello V1 . Hepatitis C virus infection and type II cryoglobulinemia: an immunological perspective. See comment in PubMed Commons below Hepatology. 1997; 26: 1375-1379.
- Fabrizi F1, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P . Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. See comment in PubMed Commons below Am J Kidney Dis. 2013; 61: 623-637.
- D'Amico G1 . Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. See comment in PubMed Commons below Kidney Int. 1998; 54: 650-671.
- Berger J, Yaneva H, Nabarra B . Glomerular changes in patients with cirrhosis of the liver. See comment in PubMed Commons below Adv Nephrol Necker Hosp. 1977; 7: 3-14.
- Poole B, Schrier R, Jani A. Glomerular disease in cirrhosis. Ascites and Renal Dysfunction in Liver Disease (2008): 360.
- Cao Y1, Zhang Y, Wang S, Zou W . Detection of the hepatitis C virus antigen in kidney tissue from infected patients with various glomerulonephritis. See comment in PubMed Commons below Nephrol Dial Transplant. 2009; 24: 2745-2751.
- Novak J1, Julian BA, Mestecky J, Renfrow MB . Glycosylation of IgA1 and pathogenesis of IgA nephropathy. See comment in PubMed Commons below Semin Immunopathol. 2012; 34: 365-382.
- Tomana M1, Kulhavy R, Mestecky J . Receptor-mediated binding and uptake of immunoglobulin A by human liver. See comment in PubMed Commons below Gastroenterology. 1988; 94: 762-770.
- Feehally J1, Allen AC . Pathogenesis of IgA nephropathy. See comment in PubMed Commons below Ann Med Interne (Paris). 1999; 150: 91-98.
- Roccatello D1, Picciotto G, Torchio M, Ropolo R, Ferro M, Franceschini R, Quattrocchio G . Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients. The role of asialoglycoprotein receptors. See comment in PubMed Commons below Lab Invest. 1993; 69: 714-723.
- Machado MV1, Cortez-Pinto H . Insulin resistance and steatosis in chronic hepatitis C. See comment in PubMed Commons below Ann Hepatol. 2009; 8 Suppl 1: S67-75.
- Mehta SH1, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M . Hepatitis C virus infection and incident type 2 diabetes. See comment in PubMed Commons below Hepatology. 2003; 38: 50-56.
- Kim JY1, Akalin E, Dikman S, Gagliardi R, Schiano T, Bromberg J, Murphy B . The variable pathology of kidney disease after liver transplantation. See comment in PubMed Commons below Transplantation. 2010; 89: 215-221.
Citation: Rossini D, Kim Y and Weber M. Quadruple Nephropathy in a Liver Transplant Patient. Austin J Nephrol Hypertens. 2015;2(2): 1034.
Citation: Bucci J and Hansen KE. Should we treat Secondary Hyperparathyroidism in Patients with Pre-Dialysis Chronic Kidney Disease?. Austin J Nephrol Hypertens. 2015; 2(4): 1046. ISSN : 2381-8964