
Research Article
Austin J Nephrol Hypertens. 2015;2(3): 1043.
Arterial Hypertension in Dialysis Patients: Impacts of Age, Gender, Dialysis Characteristics and Etiologies of Chronic Kidney Diseases
Naseri M*
Department of Pediatric Nephrology, Mashhad University of Medical Sciences, Iran
*Corresponding author: Mitra Naseri, Department of Pediatric Nephrology, Dr. Sheikh Children Hospital, Mashhad University of Medical Sciences, Naderi Street, Taabodi Avenue, Mashhad, Iran
Received: July 14, 2015;Accepted: August 10, 2015;Published: August 12, 2015
Abstract
Hypertension is common in chronic kidney diseases. The study was done in a six month period. Totally 77 dialysis cases including 39 (50.6%) peritoneal dialysis and 38 (49.4%) hemodialysis patients enrolled the study. in overall 38 cases (49.4%) had arterial hypertension. In 17 (22.1%) and 22 patients (28.5%) blood pressures were in normal or prehypertension ranges respectively. In hypertensive patients there was a shorter time from onset of dialysis (P=0.054), and also glomerular disorders as etiology of chronic kidney diseases were more prevalent (P=0.057), but no significant differences were found based on gender, modality of dialysis, anuria and non-anuria conditions and characteristics of dialysis (hours of dialysis weekly in HD and volume of dialysis daily (cc/kg/ day) in CAPD cases (P>0.05 for all). We found that hypertension is a common complication of CKD, patients and dialysis characteristics and also etiology of CKD didn’t have significant impact on prevalence of hypertension.
Keywords: Arterial hypertension; Modality of dialysis; Etiology of CKD; Children; Adults
Introduction
Hypertension is a common complication of chronic kidney diseases (CKD); about 38% of children with CKD in the United States are receiving antihypertensive therapy [1]. The frequency of hypertension in adults with CKD is much higher, and more than 80% of patients with CKD are hypertensive [2]. Despite dramatically improvement of mortality rates in pediatric cases with end stage renal diseases (ESRD), mortality rates are still 30 times higher than normal pediatric population and cardiovascular diseases account for majority of death [3]. Beside to cardiovascular complications, hypertension is one of the most important factors that contribute to progression of kidney disease toward ESRD [2]. The primary goal of the study was to identify the prevalence of arterial hypertension in dialysis patients and the secondary goals were to compare hypertensive with non-hypertensive cases based on patients and dialysis characteristics and also etiology of CKD to identify the impacts of these factors in prevalence rates of hypertension.
Materials and Methods
This study was conducted in hemodialysis (HD) and peritoneal dialysis (PD) sections of an academic tertiary hospital center from January 2010, to June 2011. Details about patients and dialysis characteristics and etiology of CKD were recorded in a designed checklist. The study was funded by a research grant from research and development section of our academic center and approved be local ethic committee.
Seventy seven CKD patients who either were placed on HD or continuous ambulatory peritoneal dialysis (CAPD) enrolled a crosssectional study. They consisted of 39 CAPD (50.6%) and 38 (49.4%) HD patients. Details of enrolled cases are presented in Table 1. The types of dialysis machines were Fresenius 4008 (Germany), AK95 and AK96 (Swiss) and the types of dialyzer were low flux dialyzer (R3-R5 and low flux Poly Sulfone membranes). The blood flow rates were regulated on 100-300 cc/min. In CAPD cases routinely peritoneal dialysis solution with 1.5% Dextrose was administrated. Figure 1 illustrates the etiologies of CKD. The most common etiology of CKD was vesicoureteral reflux (VUR). Three type’s categorizations were used in enrolled cases:
Characteristics of the patients
Age ; 4 months - 25 years (123.5± 88.9 months )
Gender; 42 boys (54.5%) and 35 girls (45.5%)
Modality of dialysis ; 37 cases(49.1% ) HD and 40 patients (51.9%)CAPD
Duration from onset of dialysis
1 month-10years and 8 months(27.2± 28.1 months )
History of transplantation
Positive history (8 cases; 10.4%)
Characteristics of dialysis in CAPD cases
Number of dialysis cycle per day
3-8(4.9±0.85)
Volume of dialysis in each cycle(cc/kg)
20-77(36.7± 12.8)
Duration of each cycle (hour)
1-4(3.1±0.95)
Characteristics of dialysis in HD patients
Dialysis session per week
1-4 (2.8± 0.76)
Duration of each session(hour)
3-4(3.6±0.5)
standard HD(12 hours/week)
14cases (37.8% )
Dialysis doses lower than standard( <12 hours/week)
20 patients (54.1 % )
Dialysis doses more than standard(>12 hours/week)
3 case (8.1 %)
Table 1: Characteristics of 77 enrolled patients.
1) Age categorization: According to the age they categorized into 5 age sub- groups:
Group 1 (= 1yr), group 2 (2-5yr), group 3 (6 -10 yr), group 4 (11- 16 yr) and group 5 (>16 yr). The number of cases in each age groups were 7 (4 boys and 3 girls), 20 (11 boys and 9 girls) 10 (8 boys and 2 girls), 18 (5 boys and 13 girls) and 22 (14 boys and 8 girls) respectively. All of cases in group 1 and majority in group 2 (17 of 20 cases; 85%) used CAPD modality, whereas majority of subjects in groups 4 and 5 [10 of 18 (55.6%), and 18 of 22 (81.8%) cases respectively] used HD modality. In group 3 there were 5 CAPD and 5 HD patients.
2) Time from onset of dialysis: based on duration from placing on dialysis cases were divided into 3 sub-groups: = 1 year, 2-5 years and >5 years from onset of dialysis.
3) According to the most prevalent etiologies of CKD cases were categorized into 4 groups:
A) VUR either primary or secondary
B) Glomerular disorders including acute (rapidly progressive glomerulonephritis) or chronic glomerulonephritis (such as lupus nephritis) and different types of nephrotic syndromes
C) Renal dysplasia (± posterior urethral valve)
D) Idiopathic cases
4) Based on arterial blood pressure (BP) levels they divided in to 2 groups: hypertensive (BP> 95 percentile for age, gender and height) and non- hypertensive cases (BP = 95 percentile for age, gender and height; this group included cases with normal and prehypertension conditions).
Our policies for management of hypertension were salt restriction, applying more ultrafiltration in hypertensive patients and adding anti-hypertensive medications. Anti-hypertensive therapies usually started with single drug preferably loop diuretics in cases with nonanuria condition and calcium channel blockers (CCB) or angiotensin converting enzyme inhibitors (ACEI) in anuria condition. Then dose of the drug was increased up to its maximal recommended dosage. In cases of facing with severe drug side effects or didn’t reach the optimal systemic blood pressure (systolic and diastolic blood pressure <90 percentile), new drug or drugs were added. In cases with severe hypertension (>99 percentile for age, gender and height) frequently multiple drugs were recommended as first line of treatment. Table 2 determines the types of antihypertensive medications which patients were receiving on the time of the study.
Types of drug
Number (%)
Loop diuretics (furosemide )
24(70.6)
Calcium channel blockers(CCB)
Short acting CCB(Nifidipine )
Long acting CCB(amlodipine)
14(41.2)
9(26.5)
Angiotensin converting enzyme inhibitors (ACEI)
(captopril /enalaprile )
7(20.6)/8(23.5)
Vasodilators
Hydralazine
5(14.7)
𝛃 blockers
Metoral
Propranolol
Atenolol
4(11.7)
4(11.7)
1(2.9)
Ganglionic blocking agents
Methyldopa
5(14.7)
Anti-angiotensin II receptor blockers (ARBs)
Losartan
4(11.7)
Combination of 2 drugs
10(29.4)
Combination of 3 drugs
10(29.4)
Combination of 4 drugs
3(8.8)
Combination of 5 drugs
1(2.9)
Combination of 6 drugs
1(2.9)
Table 2: Types of antihypertensive drugs recommended for 34 hypertensive patients.
Blood pressure measurement was done by auscultatory method. Blood pressure Cuffs used in children have various sizes, often labeled neonatal, infant, pediatric, small adult, adult, and large adult or thigh cuff [4]. Different characteristics for suitable bladder cuff size have been considered [5,6]. We used a bladder cuff which at least covered two third of upper arm length (UAL) as used in some studies cited by the Task Force [7,8]. In HD cases blood pressure and weight routinely were measured before and after each dialysis sessions, while in CAPD cases they were checked monthly except in hypertensive cases who recommended repeated blood pressure control monthly. Normal, prehypertension and hypertension were considered as systolic or diastolic blood pressure levels below 90 percentile, 90- 95 percentile and more than 95 percentile for age, gender and height in age group =18 year respectively [9]. In adults (>18 years) normal, prehypertension and hypertension ranges for systolic blood pressures were defined as <120mmHg, 120-139 mmHg and =140mmHg respectively. Diastolic blood pressures <80mmHg, 80- 89 mmHg and =90mmHg were considered normal, prehypertension and hypertension respectively [10]. Mean systolic and diastolic blood pressures in a one-month period (HD cases) and recorded systolic and diastolic blood pressures in a one-month visit (CAPD cases) were assessed in the study.
The primary purpose of the study was to identify the prevalence of arterial hypertension in HD and CAPD children and young adults who were placing on dialysis. The secondary purpose was to define whether factors such as age, gender, dialysis modality, time from onset of dialysis, etiology of CKD and hours of dialysis per week in HD and total volume of infused peritoneal dialysis solution daily (cc/kg/daily) have any significant impacts on prevalence rate of hypertension. Chi square and independent T tests were used for data analysis. Univariate analysis was performed to compare different clinical and laboratory variables in patients. For all of these tests P-values <0.05 were considered as significant statistical differences.
Results
Totally 35(45.5%) girls and 42 boys (54.5%) enrolled the study. The ages of enrolled cases were 4 months to 25 years (123.4±88.9 months). They were placed on dialysis from one month to 10 years and 8 months ago (27.2±2.8 months). Of 77, 38 cases (49.4%) had arterial hypertension, 34 were taken antihypertensive medications and 4 cases were not receiving antihypertensive drugs (newly diagnosed or missed cases). Both systolic and diastolic blood pressures were in prehypertension ranges in 20 of 77 ( 26%) cases, in 17 case normal systolic and diastolic blood pressures were noted (without using antihypertensive drugs), and in the reminder (2 patients; 2.6%) either systolic or diastolic blood pressures were in prehypertension ranges.
Table III presents the BP status at the time of the study in hypertensive cases. with normal or prehypertension), there was a shorter time from onset Table 3 defines the ranges of blood pressure and prevalence of hypertension in different age groups of patients, and Figures 2A-2E illustrate systolic and diastolic blood pressures in different age groups.
Age group
(N/%)
Systolic blood pressure(mm/Hg)
[Minimum-maximum(mean )]
Diastolic blood pressure(mm/Hg)
[Minimum-maximum(mean )]
Prevalence of hypertension (%)
=12 months (7; 9.1%)
80-120(85.7)
40-75(49.3)
4 of 7 cases (57.1%)
2-5 years (20; 26%)
70-160(85.5)
40-80(54.5)
7 of 20 cases (35% )
6-10 years (10; 13%)
90-15(118.5)
40-90(72.4)
8 of 10 cases ( 80% )
11-16 years (18; 23.4%)
80-160(112)
50-100(68)
6 of 18 cases (33.3%)
>16years (22; 28.5%)
80-170(117)
40-110(72.9)
13 of 22 cases (59.1% )
Table 3: Ranges of systolic &diastolic blood pressures and prevalence of hypertension in different age groups of enrolled cases.
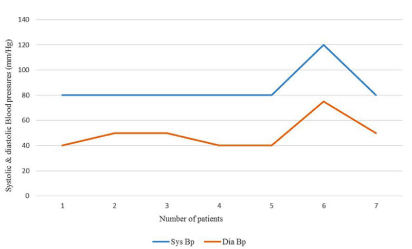
Figure 2A: Systolic and diastolic blood pressures in age group =1 year.
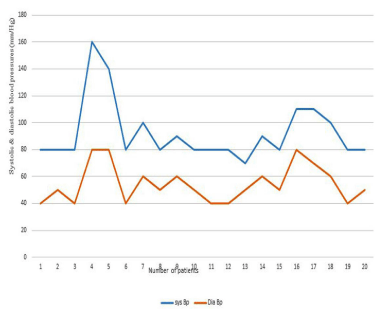
Figure 2B: Systolic and diastolic blood pressures in age group 2-5 years.
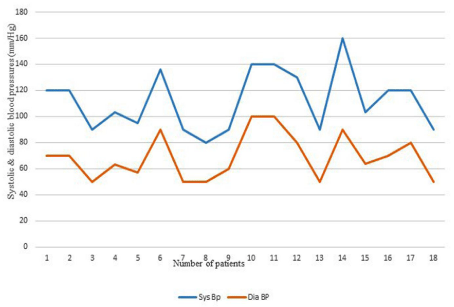
Figure 2C: Systolic and diastolic blood pressures in age group 6-10 years.
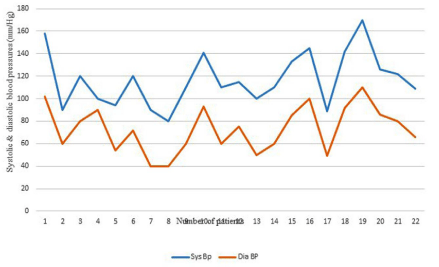
Figure 2D: Systolic and diastolic blood pressures in age group11-16 years.
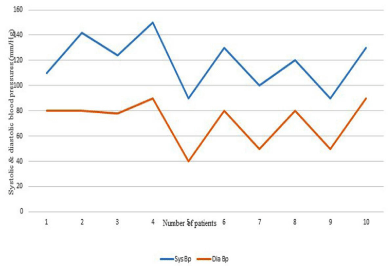
Figure 2E: Systolic and diastolic blood pressures in age group >16 years.
Comparing hypertensive with non-hypertensive cases (those with normal or prehypertension), there was a shorter time from onset of dialysis in hypertensive patients (P=0.054), and also glomerular disorders as etiology of CKD were more prevalent (P=0.057), and also no significant difference based on gender, modality of dialysis, anuria and non-anuria conditions were noted (P>0.05 for all) (Table 4). Also there were no significant differences between different age subgroups based on presence or absence of arterial hypertension (P=0.102).
Characteristic
Hypertensive cases
(38; 49.4%)
Non- Hypertensive cases
(39; 51.6%)
P-value
Age(month)1
130.7±88.4
116.5±89.9
0.486
Duration from onset of dialysis1
20.93±20.89
33.6±33
0.054
Hours of dialysis /week in HD cases1 (hours/week)
10.5±2.6
9.7±3
0.402
Dialysis volume /day in CAPD cases1 (cc/kg/day)
180.3±62.3
172.4±71.5
0.723
Standard HD (12 hours weekly)2
Non- Standard HD(<12 hours weekly)3
8(53.3%)
10(52.6%)
7(46. 7%)
9(47.4%)
0.968
Gender2
Male
Female
18(42.8%)20(57.2%)
24(57.2%)
15(42.8% )
0.212
Modality of dialysis2
HD
CAPD
20(52.6%)
18(46.2%)
18(47.4%)
21(53.8%)
0.57
Urine output status2
Anuria
Non –anuria
9(64.3%)
26(45.6%)
5(35.7%)
31 (54.4%)
0.211
Etiologies of CKD2
VUR(primary and secondary forms)
Glomerulopathies
Renal dysplasia
Idiopathic
13(40.6%)
11(84.6%)
3(42.8%)
9(56.3%)
19(59.4%)
2(15.4%)
4(57.2%)
7(43.7%)
0.057
Values are presented as number (%) or mean standard deviation.1: Student’s t-test, 2: Chi square test. 3) In 3 case hours of dialysis weekly were more than standard (>12 hours weekly)
Table 4: Blood pressure ranges in hypertensive cases at time of the study.
Based on the time from onset of dialysis cases were categorized into 3 sub-groups: group 1 (= 1yr), group 2 (2-5yr) and group 3 (>5 yr). Seventeen of 29 (58.6%) cases in group 1, 17 of 34 (50%) subjects in group 2 and 3 of 10 (30%) patients in last group were on hypertensive medications on the time of the study (P=0.294). Onsets of dialysis were not recorded clearly in 4 patients. Mean number of anti-hypertensive drugs which each sub-groups were receiving at the time of the study were 2.2, 2.3 and 3 drugs respectively. Mean number of anti-hypertensive medications that were recommended for blood pressure control in cases with anuria and those without anuria were 2.9 and 2.2 respectively (p=0.054). Cases with glomerulopathy needed more antihypertensive drugs for blood pressure control [3 medications versus 2, 2 and 2.1 drugs in cases with VUR, renal dysplasia and those with idiopathic etiology respectively (P=0.158)]. Details about CAPD and HD cases are presented in Table 5.
Characteristics
HD1 cases (N/%)
(38 ;49.4 )
CAPD2 cases (N/%)
(39; 50.6 )
Total number
Age group
= 12 months
1-5 years
6-10 years
11-16 years
>16 years
0
2(10)
5(50)
11(61.1)
20(90.9)
7(100)
18(90.)
5(50)
7(38.9)
2(9.1)
7
20
10
18
22
Gender
Male
Female
21(55.2)
17(44.8)
21(53.8)
18(46.2)
42
35
History of transplantation
Yes
No
6(15.8)
32(84.2)
2(5.1)
37(94.9)
8
69
Urine output status3
Anuria
Non anuria
8(57.1)
25 (43.9)
6(42.9)
32 (56.1)
14
57
Mean systolic blood pressure (at the time of study )
Normal
Prehypertension
Hypertension
17(36.2)
6(75)
15(68.2)
30(63.8)
2(25)
7(31.8)
47
8
22
Mean diastolic blood pressure (at the time of study )
Normal
Prehypertension
Hypertension
19(50)
6(15.8)
13(34.2)
26(66.7)
5(12.8)
8(20.5)
Main etiologies of CKD
VUR
Glomerulopathies
Renal dysplasia
Idiopathic
16(50 )
7(53.8 )
0(0)
10(62.5 )
16(50 )
6(46.2)
7(100)
6(37.5)
32
13
7
16
Total hypertensive cases4
20(52.6)
18(46.2)
38
1) Hemodialysis 2) Continuous ambulatory peritoneal dialysis 3) Urine output status in 6 cases were not clear 4) All case with arterial hypertension and also including those who have receiving anti-hypertensive medication and had normal blood pressure at the time of the study
Table 5: Comparing clinical and dialysis characteristics in hypertensive versus non-hypertensive cases.
Discussion
Hypertension is one of the most preventable causes of death and morbidity in CKD cases. The prevalence of hypertension is high in adult patients with CKD (87–90%) [11], in a study the prevalence of complications that occurred in children with CKD were assessed [12]. The study and 4 patients (5.2%) who didn’t receive antihypertensive.
Hypertension was the most common complication in all five stages of CKD (70.2%). Recent data from USA indicate that about 38% of children with CKD in the United States are receiving antihypertensive therapy [13]. In our cases which include patients with CKD stage 5, 34 patients (44.2%) were on antihypertensive therapy at the time of drugs needed these medications. Toto reported a prevalence of 80% for hypertension in adults with CKD [2]. In our series 12 cases (15.6%) were adult patients (>18 years), their ages were 18yr and 4 months up to 25 yr (mean 20 yr and ten months). Six of adults (50% of adult cases) had arterial hypertension and were on anti-hypertensive medications.
Children with certain underlying diseases like glomerular and polycystic kidney disease, are more prone to develop hypertension [14,15]. Inappropriate normal renin levels with considering the degree of hypertension and volume overload [16,17], sodium retention and hyperreninemia in cases with renal cysts and scars, microangiopathic lesions or tubulointerstitial inflammation [18,19] are factors participating in pathogenesis of hypertension in CKD. Residual urine output has been reported lower in hypertensive children on dialysis rather than their normotensive peers [15]. This finding and lowers mean blood pressure after nephrectomy despite causing anuria [20] point to presence of important volume independent factors for blood pressure regulating in HD patients. Increase in both plasma renin activity and catechol amines after HD were suggested as important factors in pathogenesis of hypertension in CKD [21].
Dietary sodium restriction, improve dialysis prescription and pharmacological treatments are main therapeutic approaches to hypertension in CKD [22]. Regression of end-organ damage (especially left ventricular hypertrophy ), lowering of blood pressure below target values with minimal drug side effects are the aims of antihypertensive therapies. A blood pressure of <130/80mmHg in adults [23,24], and <90th percentile in children [25] are recommended as goals of antihypertensive treatments in CKD.
Control of extracellular volume is a key parameter for reducing the arterial hypertension. Plum et al. [26] compared body fluids distribution between HD and PD adults by using some biophysical and biochemical methods. They found that total body water is increased in PD patients. In addition they showed links between extracellular expansion and elevated systolic blood pressure. Clinical signs of over hydration were significantly more common in PD versus HD cases (87.5±35.3% versus 53.9±50.8%), and systolic blood pressure was significantly higher in PD cases than HD patients (162±21 versus 148±30). In contrast to their findings we found higher prevalence of arterial hypertension in HD compared to CAPD cases (52.6% versus 46.2%) (P= 0.57) (Table 5).
During the first few months or years of dialysis, while residual renal function (RRF) and diuresis are maintained, PD patients usually provide better fluid and blood pressure control than HD [27-29] probably due to better saving the urine output, continuous ultrafiltration or better clearance of vasopressor toxins [30]. Several years after starting PD, the prevalence of hypertension increases in PD cases as a result of progressive loss of RRF and decreased peritoneal membrane capabilities [31-34]. Our findings in HD and CAPD patients didn’t confirm such association between time from onset of dialysis and prevalence of arterial hypertension. In overall prevalence of hypertension one and 2-5 years after onset of dialysis in CAPD cases were 52.9% and 40% respectively and just 2 cases were placed on dialysis from >5 years ago who had a normal blood pressure without using antihypertensive medications (P=0.483). In contrast to previous reports [31-34] our CAPD cases had reached a better blood pressure control with increasing time from onset of dialysis.
In HD cases who were placed on dialysis from = 1yr, 2-5 yr and >5 yr, the prevalence of arterial hypertension were 63.6%, 69.2% and 33.3% respectively (P=0.217). Unlike other reports [27-29] HD patients who were received dialysis from more than 5 years had the lowest prevalence of hypertension. This trend of changes in prevalence of hypertension was not related to changes in urine output. With increasing the time from onset of dialysis in HD cases, the anuria condition gradually increased as in first year of hemodialysis 10%, in 2-5 years of dialysis 23% and after 5 years 33% of HD cases reached anuria condition, while the highest prevalence of hypertension was found in those who were dialyzed from 2-5 years ago, and those who were placed on HD from >5 years ago had the lowest prevalence of hypertension.
In CAPD cases prevalence of anuria didn’t change significantly 2-5 years after onset of dialysis (15%) compared with first year of dialysis (17.6%). We didn’t measure the RRF in cases without anuria. What were the reasons for these paradoxical changes in urine output with increase in time from onset of dialysis in CAPD subjects? It’s not clear.
Prevalence of hypertension in our cases (patients with CKD stage V) was 2/3 of that reported by Wong et al. (who consisted of different stages of CKD) [8]. This finding was surprising since it was expected that in end stage renal failure (CKD stage V) which is associated with the highest decrease in glomerular filtration rates (GFR), signs of sodium retention and fluid overload increase. This difference in prevalence of hypertension in our subjects compared with patients evaluated by Wong et al. may be related to higher frequency of glomerular disorders in their cases compared with our patients (27.2% versus 14.3%).
Higher frequency of hypertension in subjects with glomerular disorders was completely expected since volume overload due to decreased GFR and increased activity of renin–angiotensin aldosterone system which are accompanying glomerular diseases results to sodium retention and finally lead to arterial hypertension. Why hypertension was more frequent in those who recently were placed on dialysis? One explanation might be that dialysis either HD or PD remove sodium and water effectively and help to prevent volume overload which finally leads to decrease in blood pressure, thus cases who were receiving dialysis recently and were hypertensive gradually change to normotensive cases.
In contrast to HD which is an intermittent procedure, CAPD is a continuous treatment of uremia and may be more advantageous for blood pressure regulation. Prevalence of hypertension is considerably lower in peritoneal dialysis patients [35]. In our cases although arterial hypertension was more prevalent in HD subjects versus CAPD cases (52.6% versus 46.2%), the difference was not significant (P=0.57). In the ESRD Core Indicators Project of 1219 CAPD patients 26% had systolic and 16% had diastolic hypertension [36]. In our study of 39 CAPD cases, 18 cases (46.2%) had histories of systolic and diastolic hypertension and were receiving anti-hypertensive drugs. At the time of study systolic blood pressures were normal in 30(76.9%) subjects including 11 cases on anti- hypertensive medications, and were in prehypertension and hypertension ranges in 2 (5.1%) and 7 (18%) cases respectively. Diastolic blood pressures were in normal, prehypertension and hypertension ranges in 26 (66.7%), 5 (12.8%) and 8 (20.5%) patients respectively. A similar extended study in HD cases [37] showed that before placing on dialysis prevalence of hypertension was considerably higher in HD than CAPD subjects, but after starting the dialysis the prevalence of arterial hypertension was comparable between 2 groups. In our cases of 19 HD patients who despite anti-hypertensive medications at the time of study had high systolic or diastolic blood pressures, 6 (31.6%) were placed on dialysis from = 1 years, whereas 13 cases (68.4%) were receiving HD from >1 year ago (P=0.831). In CAPD population of 7 cases who despite anti-hypertensive treatments had high blood pressures, 3 (42.9%) were placed on dialysis from = 1 year and 4 (57.1%) were receiving PD from > 1 year (P=0.791).
The main limitation of the study was that monograms of systolic and diastolic blood pressures in USA children were used for definition of normal blood pressure, prehypertension and hypertension. Unfortunately standard published monograms blood pressures in Iranian children were not accessible.
Conclusion
We found that arterial hypertension is a common complication of CKD stage 5 (49. 3%). The highest and lowest prevalence of hypertension were seen in school aged children (6-10 years; 80%) and early years of teenager period (11-16 years; 33.3%) respectively. In addition we found it’s as common in children as young adults, and hemodialysis as CAPD patients. Patients with glomerular diseases and those who recently were placed on dialysis are more prone to develop hypertension. Hypertension is as prevalent in cases with anuria as those without. It is not affected by hours of dialysis weekly in HD cases and also dialysis solution volume applied per day (cc/kg/ day) in CAPD cases. In addition HD cases on standard dialysis (12 hours weekly) are affected by arterial hypertension as cases with lower weekly dialysis doses (<12 hours weekly). Absence of significant effects of dialysis characteristics on prevalence of hypertension suggest that may be renin dependent factors are more important in pathogenesis and maintaining high arterial blood pressure in dialysis cases.
Absence of significant effects of dialysis characteristics on prevalence of hypertension suggest that renin dependent factors are more important in pathogenesis and maintaining high arterial blood pressure in dialysis cases, Thus it seems that ACEI medications should be considered as first line antihypertensive drugs in treatment of hypertension in dialysis subjects. Whether using ACEI medications as first line of treatment instead of loop diuretics can improve BP control in dialysis cases (those with and without anuria) is a good question that should be answered by more studies.
Acknowledgement
The author would like to appreciate Dr. Esmaeeli, Dr. Ghaneh and Dr. Azarfar (pediatric nephrologists) for their advocated helps.
References
- McConnell KJ, Stadler SL. Update on the Therapeutic Management of Hypertension. ACSAP. 2014; 10-32.
- Toto RD. Treatment of hypertension in chronic kidney disease. Semin Nephrol. 2005; 25: 435-439.
- Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA. 2013; 309: 1921-1929.
- Arafat M, Mattoo TK. Measurement of blood pressure in children: recommendations and perceptions on cuff selection. Pediatrics. 1999; 104: e30.
- [No authors listed]. Report of the Second Task Force on Blood Pressure Control in Children--1987. Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987; 79: 1-25.
- [No authors listed]. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996; 98: 649-658.
- Fixler DE, Laird WP. Validity of mass blood pressure screening in children. Pediatrics. 1983; 72: 459-463.
- Zinner SH, Rosner B, Oh W, Kass EH. Significance of blood pressure in infancy. Familial aggregation and predictive effect on later blood pressure. Hypertension. 1985; 7: 411-416.
- Brewer ED. Evaluation of hypertension in childhood diseases. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, editors. Text book of pediatric nephrology. 6th edn. Springer. 2009; 1459-1485.
- U.S. department of health and human services. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003; 42: 1206-1252.
- Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003; 16: 101-105.
- Wong H, Mylrea K, Feber J, Drukker A, Filler G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006; 70: 585-590.
- Swinford RD, Portman RJ. Measurement and treatment of elevated blood pressure in the pediatric patient with chronic kidney disease. Adv Chronic Kidney Dis. 2004; 11: 143-161.
- Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol. 2003; 14: 2618-2622.
- Tkaczyk M, Nowicki M, Balasz-Chmielewska I, Boguszewska-Baçzkowska H, Drozdz D, Kollataj B, et al. Hypertension in dialysed children: the prevalence and therapeutic approach in Poland--a nationwide survey. Nephrol Dial Transplant. 2006; 21: 736-742.
- Bras H, Ochs HG, Armbruster H, Heintz R. Plasma renin activity (PRA) and aldosterone (PA) in patients with chronic glomerulonephritis (GN) and hypertension. Clin Nephrol. 1976; 5: 57-60.
- Warren DJ, Ferris TF. Renin secretion in renal hypertension. Lancet. 1970; 1: 159-162.
- Loghman-Adham M, Soto CE, Inagami T, Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2004; 287: F775-788.
- Ibrahim HN, Hostetter TH. The renin-aldosterone axis in two models of reduced renal mass in the rat. J Am Soc Nephrol. 1998; 9: 72-76.
- Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001; 12: 2427-2433.
- Rauh W, Hund E, Sohl G, Rascher W, Mehls O, Schärer K. Vasoactive hormones in children with chronic renal failure. Kidney Int Suppl. 1983; 15: S27-33.
- Hadtstein C, Schaefer F. Hypertension in children with chronic kidney disease: pathophysiology and management. Pediatr Nephrol. 2008; 23: 363-371.
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004; 43: S1-290.
- Chobanian AV, Barkis GL, Black DL, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003; 289: 2560– 2571.
- National high blood pressure education program working group on high blood pressure in children and adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescent. Pediatrics. 2004; 114: 55 5–576.
- Plum J, Schoenicke G, Kleophas W, Kulas W, Steffens F, Azem A, et al. Comparison of body fluid distribution between chronic haemodialysis and peritoneal dialysis patients as assessed by biophysical and biochemical methods. Nephrol Dial Transplant. 2001; 16: 2378-2385.
- Hamburger RJ, Christ PG, Morris PA, Luft FC. Hypertension in dialysis patients: does CAPD provide an advantage? Adv Perit Dial. 1989; 5: 91-96.
- Lameire N. Cardiovascular risk factors and blood pressure control in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1993; 13 Suppl 2: S394-395.
- Dasgupta I, Burden R. Blood pressure control before and after starting dialysis. Nephron Clin Pract. 2005; 99: c86-91.
- Bianchi G. Hypertension in chronic renal failure and end-stage renal disease patients treated with haemodialysis or peritoneal dialysis. Nephrol Dial Transplant. 2000; 15 Suppl 5: 105-110.
- Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001; 16: 2207-2213.
- Ates K, Nergizoglu G, Keven K, Sen A, Kutlay S, Ertürk S, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001; 60: 767-776.
- Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001; 16: 1459-1464.
- Konings CJ, Kooman JP, Schonck M, Struijk DG, Gladziwa U, Hoorntje SJ, et al. Fluid status in CAPD patients is related to peritoneal transport and residual renal function: evidence from a longitudinal study. Nephrol Dial Transplant. 2003; 18: 797-803.
- Cheigh JS, Kim H. Hypertension in continuous ambulatory peritoneal dialysis patients: what do we know and what can we do about it? Perit Dial Int. 1999; 19 Suppl 2: S138-143.
- Department of Health and Human Services. An annual Report. ESRD Core Indicators Project. December. 1997.
- Department of Health and Human Services. Annual Report. ESRD Core Indicators Project. January 1997.