
Research Article
Austin J Nephrol Hypertens. 2015; 2(5): 1049.
Frequency of ESA Dose Adjustment: A Novel, Independent Predictor of Survival in HD Patients
Amidone M¹*, Dattolo PC¹, Lenti C², Betti G³, Conti P4, Panichi V5, Mannarino A6, Rosati A7 and Pizzarelli F¹
¹Department of Nephrology Units, SM Annunziata Hospital, Italy
²Department of Nephrology Units, Degli Infermi Hospital, Italy
³Department of Nephrology Units, Massa Hospital, Italy
4Department of Nephrology Units, Misericordia Hospital, Italy
5Department of Nephrology Units, Versilia Hospital, Italy
6Department of Nephrology Units, S Giovanni di Dio Hospital, Italy
7Department of Nephrology Units, Campo di Marte Hospital, Italy
*Corresponding author: Marco Amidone, Department of Nephrology Units, SM Annunziata Hospital, 58, Antella Street, Florence, Italy
Received: September 09, 2015; Accepted: November 10, 2015; Published: November 12, 2015
Abstract
Background: While hazards linked to high dosages of Erythropoietin Stimulating Agents (ESA) are extensively studied, the potential harm of ESA prescription modality is uncertain.
Methods: Dosing patterns of ESA and anemia related parameters were collected monthly during 2008 calendar year and all-cause mortality was assessed in the following year in prevalent in-centre Hemodialysis (HD) patients from 7 Nephrology Units in Tuscany, Italy.
Results: During the observation year, monthly Hemoglobin (Hb) levels and weekly ESA dose were 11.40±0.70 gr/dl and 8425±5128 IU, respectively, in the 366 subjects recruited. The higher the ESA dose the lower the Hb values (r - .38, p <0.0001). During the 1-year follow-up 13% of patients died. In comparison with those who eventually died, survivors had significantly higher ESA changes (p .03) and comparable Hb values and ESA dosages. According to Kaplan- Meier subjects with 6 or more ESA changes had significantly better survival (Log Rank 4.94, p .025) than those with less than 6 ESA changes. In a Cox model adjusted for demography, number of co-morbidities and biochemistry covariates, frequency of ESA dose change was highly and independently associated with better survival [HR for mortality 0,79 (95% CI 0,67 - 0,94), p .01].
Conclusion: Frequency of ESA dose changes is a novel independent predictor of survival among HD patients.
Keywords: Anemia; Erythropoietin; Hemodialysis; Survival
Introduction
There is evidence that high Hemoglobin (Hb) levels are associated with an increased mortality risk both in CKD as well as in hemodialysis (HD) patients [1-5]. Among the hypotheses for the explanation of increased risk linked to higher Hb targets, the potential role of Erythropoietin Stimulating Agents (ESA) dosages has been suggested [6-9]. Noteworthy, this untoward ESA effect counter stands against the finding that higher Hb concentration naturally occurring by endogenous erythropoietin does not increase mortality among HD patients [10]. Following the early 2000s observations on Hematocrit [11] and Hb [12] serum fluctuations (Hbvar) in HD patients, a number of studies have pointed to Hbvar as additional risk factor for adverse clinical outcomes including mortality [13-17]. However the association has been confuted by others [18] and it has been suggested Hbvar possibly represents just a surrogate of disease severity [19]. Anemia treatment in HD subjects is based on periodic measurements of serum Hb over time with subsequent adjustments of ESA dosages. Very few studies, to the best of our knowledge, have specifically evaluated the clinical relevance of ESA prescription modality [20]. Hence, the independent association between ESA dosing patterns and survival remains to be determined.
With this background, we aimed at evaluating whether dosing patterns of ESA are independent predictors of all-cause mortality among HD patients. Specifically we probed survival prediction of frequency of ESA dose adjustments as possible marker of good clinical practice.
Materials and Methods
Study design
This is a multicentre 2 year retrospective observational cohort study. Time-varying anemia related parameters were collected during 2008 calendar year and all-cause mortality was assessed in the subsequent year.
Following a preceding clinical audit on anemia management [21], seven HD Centers in Tuscany Region of Italy took part in this study. Although no formal protocol was implemented, all Centers shared the practice of monthly control of serum Hb values and, accordingly, promptly adjust ESA dosages with the following targets: serum Hb values 10.5-12 g/dl and amplitude of ESA dose change around 30% of the previous ESA prescription. ESA changes were performed taking into account both punctual Hb values as well as serum Hb trends.
Patients
We enrolled prevalent adult (>18y) uremic on in-centre HD treatment alive on 31.12.2008. Subjects had to have at least 9 of the 12 scheduled Hb values and at least 9 documented ESA dosages throughout 2008 calendar year. Moreover, they should have been on HD and ESA treatment at least since June 2007, e.g. 6 months before 2008 observation year, to avoid Hb and ESA variability linked to HD initiation and resulting ESA titration.
Data collection
Demographic, co-morbidities, clinical and biochemical covariates were collected from subject clinical notes and assessed at the study entry. Pre-existent co-morbidities were categorized as cardiovascular (including, cardiac, cerebral and peripheral vascular involvement), pulmonary, hepatic and tumoral (solid and hematopoietic, including myeloma); diabetes, inflammation and/or malnutrition were also recorded. Biochemical covariate data missing at baseline were imputed as the more recent registered for that subject in the following 3 months.
Frequency of ESA dose changes (ESAc) was the count in each subject of every monthly registered ESA dosage different from the previous one. Amplitude of ESA dose adjustment (ESAvar) was defined as intra-patients’ standard deviation of all the values collected in the observation year.
Intra-patient standard deviation of Hb values registered was assumed as a proxy of Hbvar. Time-varying average ESA dosages (ESAx) were expressed in IU administered weekly. ESA molecules were Darbepoetin α (Darbe) or Epoietin α or β (EPO) and no shift among molecules was allowed. To compare Darbe with EPO, the correction factor 1 μg: 200 IU was adopted, as indicated by manufacturer. According to prescription, at the end of HD sessions nurses injected intravenous Darbe 2 or 4 times per month or EPO 1 - 3 times a week.
Statistics
Data were handled according to the Declaration of Helsinki and the Italian legislation (Guarantee privacy law 6 August 2008, n. 133 and subsequent amendments), and statistically analyzed by SPSS package. Data are reported as mean±Standard Deviation (SD) or median and Interquartile Range [IQR]. Univariate estimates of the associations between time varying anemia related parameters were explored by simple linear regression by calculating the Pearson product moment correlation coefficient. Comparisons between dead and alive subjects at the end of follow up were made by ANOVA. Survival discriminating power of ESAc was assessed by log rank statistics for Kaplan-Meier survival curves. Impact of time-varying Hb and ESA related parameters on all-cause mortality as outcome parameter was evaluated by Cox proportional hazards regression modeling adjusted for a case-mix covariate including baseline demography, co morbidity and biochemistry.
Results
We analyze the 366 subjects fulfilling the selection criteria. Covariate data missing at baseline were less than 4% for each given variable, but CRP 10%. The overwhelming majority of the cohort, e.g. 350 out of 366 subjects, had 100% of longitudinal repeated anemia related parameters with the remaining 16 subjects with 10 or 11 scheduled measures.
Baseline characteristics of the cohort are shown in table 1. This is a typical Italian HD population, elderly, with relevant co-morbidities, fairly well nourished and Fe replenished, with only 25% of subjects with iPTH levels above 330 pg/ml. On average, Hb values were within targets. Fifty-six subjects, e.g. 15%, were on Darbe.
Mean ± SD
Median [25-75 IQR]
Age (yrs)
67.3 ± 13.5
70 [59.2 - 77]
Weight (Kg)
69.2 ± 15.6
67 [58 – 78.5]
BMI (Kg/m2)
25.2 ± 4.9
24.5 [21.9 – 27.4]
Co morbidities (n)
1.7 ± 1.0
2 [1 - 3]
Dialysis vintage (yrs)
6.4 ± 6.7
4.4 [2 – 8.1]
Hb (g/dl)
11.45 ± 1.16
11.4 [10.7 – 12.2]
Albumin (g/dl)
3.8 ± 0.4
3.8 [3.6 – 4.1]
CRP (mg/dl)
1.39 ± 5.41
0.55 [0.30 – 1.22]
Ferritin (ng/ml)
550 ± 396
477 [287 - 732]
Fe sat (%)
32.2 ± 15.2
28 [22 – 37.3]
PTH (pg/ml)
248 ± 220
211 [85 - 329]
Table 1: Baseline characteristics of the cohort.
During the 1-year follow-up 48 out of the 366 patients died (13%) and 13 (3.6%) were transplanted. In tab 2 are reported time-varying anemia-related parameters in the whole cohort and according to vital status at the end of the follow-up. The majority of patients, e.g. 78%, had within target Hb values with the remaining 8% and14% below and above Hb targets, respectively. This result was obtained administering average low EPO dosages (Table 2). Interestingly enough, the higher the ESAx the lower the Hbx (r- .38, p <0.0001). Nephrologists changed ESA dosages quarterly, on average, and this practice did not change according to whether patients were on Darbe or EPO (ESAc 3.07±2.01 and 3.03±1.98, respectively, p NS). The amplitude of ESA dose change (Table 2) was quite low and with quite narrow inter-subject data dispersion, such as in 75% of subjects the ESA change did not exceed 3100 IU/week. In comparison with those who eventually died, survivors had significantly higher ESAc and tended to have lower Hbvar (p =0.08). By contrast, average Hb values and ESA dosages were pretty super-imposable in both groups (Table 2).
Total
Mean±SD
Live
Mean±SD
Dead
Mean±SD
p
Hbx (g/dl)
11.40±0.70
11.39±0.71
11.42±0.67
ns
Hbvar (g/dl)
0.92±0.38
0.91±0.36
1.01±0.45
ns
ESAx (U/wk)
8425±5128
8458±5038
8209±5718
ns
ESAvar (U/wk)
2281±1774
2346±1739
1871±1952
ns
ESAc (changes/yr)
3.05±2.00
3.14±2.00
2.46±1.89
0.03
Table 2: Time-varying anaemia related parameters.
As to baseline covariates, age, co-morbidities and CRP were highly significantly higher (p< 0.0001) in those who died compared with survivors.
According to Kaplan-Meier, the 44 subjects with 6 or more ESAc in the observation year had significantly better survival than the remaining 322 with less than 6 ESAc (Figure 1).
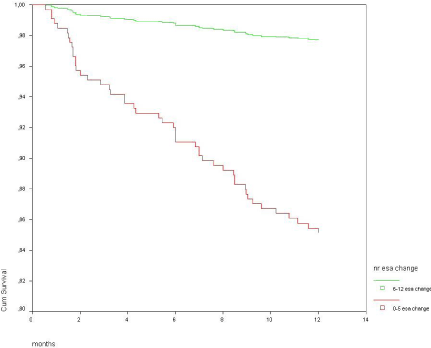
Figure 1: Kaplan-Meier survival curves according to whether ESA dose was
changed more or less than six times a year.
Log Rank 4.94, p = 0.026
The final Cox model is reported in table 3. Frequency of ESA dose change was highly and independently associated with better survival, while age, CRP and number of co morbidities were strongly associated with mortality. Hb variability, too, was a strong and independent predictor of mortality while both time-varying average Hb values and ESA dosage were not.
HR [95% CI]
p
Age, per 1 year
1.06 [1.03 – 1.10]
0.00
Comorbidities, per 1 comorbidity
1.79 [1.22 – 2.62]
0.00
CRP, mg/dl
1.03 [1.01 – 1.05]
0.01
ESAc, per 1 ESA dose change
0.79 [0.67 – 0.94]
0.01
Hbvar, per 1 SD
2.43 [1.17 – 5.02]
0.02
Table 3: Final Cox model.
Discussion
Main result of our study is that in HD subjects the higher the frequency of ESA dose adjustments, the lower the mortality. This association was robust and independent of a panel of potential confounders, particularly so when ESA dosage changes were performed 6 times a year or more, that is to say at least every other month. Interestingly enough, Hb variability predicted mortality while average Hb value and ESA dose did not.
We enrolled prevalent HD subjects stabilized in ESA since a long time. Their demographic, clinical and biochemical case-mix and relatively low gross mortality are in line with corresponding figures in Tuscany HD population. Following agreements of an audit on anemia management [21], participating Centers in this study shared the clinical practice of small and tailored ESA dose adjustments. Accordingly, the 2008 time-varying average ESA dose prescribed was quite low and the inter-subject data dispersion quite narrow. Our prescriptive approach was an attempt to minimize the “un-physiology” of ESA treatment, characterized by an abrupt rise of serum EPO after injection followed by rapid decline, as opposed to the normal biology of endogenous EPO secretion [7]. Anemia associated with chronic diseases, such as renal failure, could be an adaptive response [22], which therefore must be corrected with caution.
To the best of our knowledge, very few papers have specifically addressed the clinical relevance of ESA prescription modality. Lau et al., [20] found that the larger the ESA dose increases the higher the mortality, while we found that frequency of ESA dose changes was a strong predictor of survival. Aside from the different study design -the former is a secondary analysis of a randomised controlled trial, our is a retrospective clinical trial-, in the Lau paper average ESA dosage and ESA dose change were 11,377 IU/week and 4000 IU/week, respectively, e.g. figures much higher than ours.
Although in our study the mean number of ESA dose change among survivors and deceased was statistically different, the clinical significance of the modest difference observed might appear trivial. However, discriminants survival analyses identified number of ESA changes as strong independent predictor of survival, particularly so when number of changes were 6 or more yearly, e.g. every other month.
Our study population consisted of a mixture of patients receiving Darbe and EPO. Hence, problem arises whether the dose changes of these two agents are comparable, given the differences in their halflives and prescription frequencies. However, the half life of Darbe, although longer than EPO, is decisively shorter than the monthly elapsing time for ESA change. Accordingly, it comes as no surprise that ESA changes among subjects on Darbe or EPO was almost superimposable. Therefore, both pharmacodynamics and results achieved legitimate data pooling.
In decision making whether or not changing ESA doses, we adopted the policy of taking into account both punctual Hb values and Hb trends with the aim of preventing too large Hb variability with its attendant untoward effects [13-17]. And indeed our Hbvar was 7.7% [IQR 5.7-10%] of average Hb values. This value is well below the limit set in defining Hb fluctuations [13] and approaches the 2% naturally occurring seasonal variations in Hematocrit observed by Cheung [11]. Not natural enough, however, as also the attenuated Hbvar of our study is still independent predictor of mortality. The simple way in which we measured Hbvar, e.g. within subject SD of Hb, allows to calculate at the bedside a parameter clinically relevant as predictor of survival, but it fails to discern patterns or directionality and cannot account for overall trends [18].
This paper suffers of all the limitations inherent to a retrospective survey on a relatively small cohort. Strengths rely on the quality of the collected data, testified by the overwhelming majority of patients having a complete data set and by the exquisitely homogeneous ESA prescription modality shared by all HD Centers participating in this study. Our good clinical practice has allowed a good quality of source data, directly retrieved and not reworked to adjust for case mix as it often happens in large registry studies.
Being clear about the strengths and weaknesses of our study, we think our results are clinically relevant in that they confirm the positive impact of a judicious ESA prescription modality. As far as frequency of ESA dose changes represents a surrogate marker of frequency of nephrologists visits, than we can conclude that the more the time spent in medical intervention the better the clinical outcomes.
References
- Besarab A, Bolton WK, Browne JK. The effects of normal as compared with low Hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998; 339: 584–590.
- Drueke TB, Locatelli F, Clyne N. Normalization of haemoglobin level in patients with chronic kidney disease and anaemia. N Engl J Med. 2006; 355: 2071–2084.
- Singh AK, Szczech L, Tang KL. Correction of anaemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006; 355: 2085–2098.
- Phrommintikul A, Haas SJ, Elsik M. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007; 369: 381–388.
- Pfeffer MA, Burdmann EA, Chen CY. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009; 361: 2019–2032.
- Regidor DL, Kopple JD, Kovesdy CP. Associations between changes in haemoglobin and administered erythropoietin-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006; 17:1181-1191.
- Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher haemoglobin targets. Clin J Am Soc Nephrol. 2007; 2: 1274-1282.
- Szczech LA, Barnhart HX, Inrig JK. Secondary analysis of the choir trial epoetin-alpha dose and achieved haemoglobin outcomes. Kidney Int. 2008; 74: 791–798.
- Kouloridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of Erythropoietin-stimulating Agents and Adverse Outcomes in CKD: A Met regression Analysis. Am J Kidney Dis. 2012; 61: 44-56.
- Goodkin AD, Fuller DS, Robinson BM. Naturally occurring higher haemoglobin concentration does not increase mortality among hemodialysis patients. J Am Soc Nephrol. 2011; 22: 358–365.
- Cheung AK, Yan G, Greene T. Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol. 2002; 13: 2345-2352.
- Lacson E Jr, Ofsthun M, Lazarus JM. Effect of variability in anaemia management on haemoglobin outcomes in ESRD. Am J Kidney Dis. 2003; 41:111-124.
- Fishbane S, Berns J. Haemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005; 68:1337-1343.
- Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane S, Feldman HI. Haemoglobin variability and mortality in ESRD. J Am Soc Nephrol. 2007; 18: 3164-3170.
- Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ: Haemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol. 2008; 3:133-138.
- Brunelli SM, Lynch KE, Ankers ED. Association of haemoglobin variability and mortality among contemporary incident hemodialysis patients. Clin J Am Soc Nephrol. 2008; 3:1733-1740.
- Boudville NC, Djurdjev O, Macdougall IC. Haemoglobin variability in non dialysis chronic kidney disease: examining the association with mortality. Clin J Am Soc Nephrol. 2009; 4: 1176-1182.
- Eckardt KU, Kim J, Kronenberg F. Haemoglobin Variability Does Not Predict Mortality in European Hemodialysis Patients. J Am Soc Nephrol. 2010; 21: 1765-1775.
- Weinhandl ED, Peng Y, Gilbertson DT, Bradbury BD, Collins AJ. Haemoglobin Variability and Mortality: Confounding by Disease Severity. Am J Kidney Dis. 2011; 57: 255-265.
- Lau JH, Gangji AS, Rabbat CG, Brimble KS. Impact of haemoglobin and erythropoietin dose changes on mortality: a secondary analysis of results from a randomized anaemia management trial. Nephrol Dial Transplant. 2010; 25: 4002-4009.
- Soffritti S, Russo G, Cantelli S, Gilli G, Catizone L. Maintaining over time clinical performance targets on anaemia correction in unselected population on chronic dialysis at 20 Italian centres. Data from a retrospective study for a clinical audit BMC Nephrol. 2009; 10: 33.
- Zarychanski R, Houston DS: Anaemia of chronic disease: a harmful disorder or an adaptive, beneficial response? CMAJ. 2008; 179: 333-337.