
Review Article
Austin J Nephrol Hypertens. 2016; 3(1): 1055.
Atypical Hemolytic Uremic Syndrome May Present as Severe Hypertension without Hemolysis or Thrombocytopenia
Tsai HM*
Department of Nephrology, iMAH Hematology Associates, University of Texas Southwestern School of Medicine, USA
*Corresponding author: Han-Mou Tsai, Department of Nephrology iMAH Hematology Associates, New Hyde Park, NY 11040, USA
Received: June 09, 2016; Accepted: July 05, 2016; Published: July 07, 2016
Abstract
In idiopathic hypertensive emergency or malignant hypertension, it is generally assumed that high blood pressures cause or contribute to target organ injuries, and its management has focused on antihypertensive drugs. However, some patients continue to develop progressive renal failure or other organ injury even when their blood pressures are reasonably controlled with antihypertensive drugs. In such cases, both hypertension and organ injuries may be the consequences of an underlying disease. Hypertension is a common complication of atypical hemolytic uremic syndrome (AHUS), which is commonly characterized by the triad of renal failure, microangiopathic hemolytic anemia (MAHA) and thrombocytopenia. Occasionally, hypertension may be the only manifestation of AHUS for years before its characteristic triad ensues. Early recognition of the correct diagnosis may help prevent serious complications such as advanced renal failure or even death.
Keywords: Atypical hemolytic uremic syndrome; Hypertensive emergency; Malignant hypertension; Microangiopathic hemolytic uremic syndrome; Thrombotic microangiopathy
Abbreviations
AHUS: Atypical Hemolytic Uremic Syndrome; MAC: Membrane Attack Complex; MAHA: Microangiopathic Hemolytic Anemia; STXHUS: Shiga Toxin Associated Hemolytic Uremic Syndrome; TMA: Thrombotic Microangiopathy; TTP: Thrombotic Thrombocytopenic Purpura
Introduction
Hypertensive emergency, or malignant hypertension, refers to severe hypertension accompanied with evidence of organ injury or dysfunction such as acute stroke, seizures, altered mental status, retinopathy, acute coronary syndrome or heart failure, aortic dissection and acute renal failure. It is generally assumed that hypertension causes or contributes to these target organ injuries.
Most cases of hypertension are believed to be idiopathic, except for a small number of cases that have an identifiable cause such as renal artery stenosis. The conventional management of idiopathic hypertension focuses on lowering the blood pressures with antihypertensive drugs. With this strategy, the annual incidence rates of all-cause mortality remain quite high, approaching 13-fold of normotensive control [1-4]. In fact, some patients continue to develop progressive renal failure despite good control of the blood pressure. Therefore, a subset of patients with severe hypertension does not do well with antihypertensive drugs alone.
It is increasingly recognized that target organ injury does not always correlate with blood pressure levels. Some patients present with profoundly elevated blood pressures but no acute end-organ injury; whereas others present with retinopathy, renal insufficiency and/or other organ injury even though their blood pressures do not meet the conventional criteria for severe hypertension. Taken together, these observations suggest that at least in some cases, injury or dysfunction of the kidney and other organs is not a direct consequence of hypertension. These patients may have a disease, e.g. atypical hemolytic uremic syndrome (AHUS), which causes both hypertension and organ injuries.
What is atypical hemolytic uremic syndrome?
Atypical hemolytic uremic syndrome (AHUS) originally referred to patients presenting with the triad of acute renal failure, microangiopathic hemolytic anemia (MAHA) and thrombocytopenia without a prodrome of hemorrhagic diarrhea [5]. While most cases of ‘typical’ hemolytic uremic syndrome have a prodrome of hemorrhagic diarrhea due to infection with Shiga toxin producing E. coli or other microorganisms (hence Shiga toxin-HUS or STX-HUS), many patients of AHUS are found to have molecular defects in one or more components of the regulators of the alternative complement pathway [6].
The complement system is an important component of the innate host defense. It comprises a classical/lectin pathway that may be triggered by immune complexes or microbial mannose, and an alternative pathway that amplifies the activation once the cascade is triggered [7]. This pathway of amplification provides burst generation of complement activation products such as C5b-9 complex (also known as membrane attack complex, MAC), C3a and C5a that help fend off infections. However, if it is left unchecked, incessant intravascular generation of complement activation products may cause endothelial injury and its consequences.
A complex and redundant system of regulators exist to control complement activation [8]. When one or more of the regulators are defective, uncontrolled intravascular complement activation may occur, leading to injury of vascular endothelial cells and thrombosis (hence thrombotic microangiopathy, TMA). The lesions of TMA, found primarily in the kidney, include endothelial cell swelling or disruption and sub-endothelial expansion that can be mucinous, onionskin or fibrotic in appearance. Thrombosis is not invariably present –it typically occurs at sites of endothelial disruption. The pathology of TMA seen with AHUS is quite different from that of thrombotic thrombocytopenic purpura (TTP), which is characterized with arteriolar and capillary thrombi comprising von Willebrand factor and platelets, with no or little evidence of endothelial injury [9].
Pathophysiology of AHUS
Although AHUS is best known for the triad of renal failure, MAHA and thrombocytopenia, hypertension is quite common. Furthermore, extra-renal complications can be quite serious or even fatal. These complications include cerebral abnormalities of posterior reversible encephalopathy syndrome and brain edema; exudative or ischemic retinopathy; cardiopulmonary abnormalities such as edema of the bronchial wall, pulmonary alveolar edema, arrhythmia, heart failure and pleural or pericardial effusions; abdominal abnormalities of intestinal wall edema, as cites and mesenteric and pancreatic edema; and edema of cutaneous soft tissues giving rise to the appearance of anasarca [8].
Renal failure, MAHA and thrombocytopenia due to AHUS
In AHUS, renal failure results from glomerular dysfunction, which may be the consequence of the direct effect of MAC and/or ischemic injury due to micro vascular thrombosis or non-thrombotic stenosis resulting from endothelial swelling and/or sub-endothelial expansion.
TMA causes MAHA because abnormal shear stress generated in stenotic or thrombosed arterioles and capillaries may inflict mechanical injury of red blood cells. Thrombocytopenia results from consumption of platelets in thrombosis. The syndrome of MAHA and thrombocytopenia is not specific for AHUS. It also occurs in TTP and other diseases that cause TMA or other types of pathology [8]. MAHA is also a common complication of intravascular devices such as prosthetic heart valves, extracorporeal membrane oxygenators, or left ventricular assist devices, all of which are associated with abnormal intravascular mechanical forces.
Hypertension due to AHUS
TMA often affects pre-glomerular arterioles. This may disrupt the regulation of renin release from the juxtaglomerular apparatus and explain why hypertension is common in AHUS. Hypertension of AHUS can be quite severe and wildly unstable, presumably due to the dynamic and often transient nature of acute lesions of TMA. When the sites of endothelial injury are few but happen to affect preglomerular hemodynamics, the pathology may be sufficient to cause abnormal renin release and hypertension but inadequate to cause renal insufficiency, MAHA or thrombocytopenia. Hence, AHUS may present only with hypertension without concurrent renal failure, MAHA and/or thrombocytopenia.
Extra-renal complications
The extra-renal complications of AHUS are often not due to TMA, which in most cases is not detectable except in the kidney. Instead, the predominant findings at autopsy are interstitial edema of the affected organs. Interstitial edema and accumulation of fluids in cavitary and alveolar spaces suggest that extra-renal complications are primarily the consequence of abnormal vascular permeability. It is assumed that abnormal vascular permeability is induced by anaphylatoxins C3a and C5a generated in the process of complement activation. Anaphylatoxins may cause abnormal vascular permeability by inducing secretion of histamine and other vasoactive mediators from basophiles and mast cells in various tissues and organs. Most extrarenal complications are readily reversible when disease activity abates.
The predominant location of TMA in the kidney suggests uncontrolled complement activation primarily occurs in the kidney. It is speculated that the renal microenvironments are conducive to complement activation. In vitro, low pH (Ham test) and electrolytes (sucrose hemolysis test) are known to induce complement activation and hemolysis of red blood cells.
The absence of TMA in other organs suggests the anaphylatoxins responsible for abnormal vascular permeability in extra-renal organs and tissues originate from the kidney. This scheme would explain why extra-renal complications of AHUS often ameliorate when a patient develops end stage renal disease and exacerbate after the patient undergoes kidney transplantation.
Hypertension as the sole manifestation of AHUS
Clinically, hypertension occurs in approximately 30%-50% of the cases of AHUS. Recently, it is further recognized that AHUS may present as hypertension for years before its true nature is recognized. This is vividly illustrated in a case that had, for more than four years, frequent bouts of severe but wildly unstable hypertension, accompanied with no thrombocytopenia and no or mild renal insufficiency and MAHA. The correct diagnosis of AHUS was recognized only when the patient eventually presented with the triad of acute renal failure, MAHA and thrombocytopenia, along with altered mental status [10]. Various regimens of anti-hypertensive drugs failed to stably control his blood pressures. In contrast, anticomplement therapy with eculizumab, a humanized monoclonal antibody of C5approved for the treatment of paroxysmal nocturnal hemoglobinuria and AHUS, led to rapid improvement of symptoms and resolution of thrombocytopenia within a week, as are expected for AHUS [11]. The blood pressures also stabilized within two weeks of the treatment, allowing discontinuation of intravenous nitroglycerin infusion. In contrast, his kidney function only began to gradually improve after 6 weeks of treatment, with his serum creatinine level slowly decreasing from a peak of 5.62 mg/dl to 2.67 mg/dl over the course of six months.
Hypertensive disorders of pregnancys
When AHUS presents as hypertension in a pregnant woman, it may be indistinguishable from gestational hypertension or preeclampsia. When the hypertension is accompanied with MAHA and thrombocytopenia, it may be mistaken to be the HELLP (hemolysis, elevated liver enzymes and low platelet) syndrome [12]. This misdiagnosis may account for the high prevalence of mutations affecting complement regulation in clinical series of preeclampsia and HELLP syndrome [13].
Diagnosis of forme fruste AHUS
Clinical vigilance is essential for recognition and diagnosis of AHUS. Diagnostic tests for the diagnosis of AHUS are not yet optimal in sensitivity, only identifying 40%-75% of patients with defective complement regulation [8]. Their turnaround times may be weeks to months, although faster turnaround is now available at some laboratories.
Clinical vigilance is essential for recognition and diagnosis of AHUS. Diagnostic tests for the diagnosis of AHUS are not yet optimal in sensitivity, only identifying 40%-75% of patients with defective complement regulation [8]. Their turnaround times may be weeks to months, although faster turnaround is now available at some laboratories.
For patients whose hypertension is difficult to control with antihypertensive drugs or accompanied with progressive renal failure or extra-renal complications, empiric therapeutic diagnosis with an anti complement drug such as eculizumab is an approach that deserves serious consideration for further investigation (Figure 1).
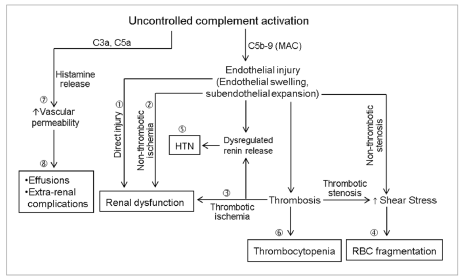
Figure 1: Multiplicity in the pathophysiology of atypical hemolytic uremic syndrome (AHUS).
In AHUS, the pathological changes of endothelial injury and thrombosis (thrombotic microangiopathy) are found primarily in the kidney, suggesting that controlled
complement activation mainly occurs in the kidney. Persistent generation of membrane attack complex (MAC, C5b-9) may lead to glomerular endothelia cell injury
and renal dysfunction (?). Renal dysfunction may also result from non-thrombotic (?) and thrombotic (?) ischemic glomerular injury. Both non-thrombotic and
thrombotic stenosis may generate abnormal shear stress in the microcirculation, resulting in fragmentation of red blood cells and hemolysis (?). These changes
may also cause abnormal renin release and hypertension (HTN) if they disrupt the pre-glomerular hemodynamics (?). Thrombocytopenia may ensue if thrombosis
causes consumption of thrombocytopenia that exceeds compensatory thrombocytopoiesis (?). In addition, anaphylatoxins C3a and C5b may be transported
to other tissues and organs, where they may cause abnormal vascular permeability (), presumably by inducing the secretion of histamine and other vasoactive
mediators from tissue mast cells and basophils. Abnormal vascular permeability is believed to underlie the common occurrence of fluid accumulation in alveolar,
pleural, pericardial and peritoneal spaces; and interstitial edema and dysfunctions of various extra-renal organs (?). When thrombotic microangiopathy is not
widespread but happens to affect renin secretion, hypertension may ensue without apparent hemolysis, thrombocytopenia or renal function impairment.
Severe hypertension may be a consequence instead of a cause of TMA
It is often assumed that severe hypertension may cause the triad of renal failure, MAHA and thrombocytopenia [2,3] and also TMA in the kidney [14]. However, similarity in clinical and pathological features suggests that some patients given the diagnosis of malignant hypertension complicated with progressive renal function impairment, MAHA and thrombocytopenia may instead have AHUS. Indeed, in a series of 14 cases with ‘malignant hypertension’, two (14%) were found to have mutations affecting the regulation of the alternative complement pathway [15].
Conclusion
AHUS may present as idiopathic hypertension, even masquerading as malignant hypertension. In pregnant women, it may be mistaken to be gestational hypertension, preeclampsia or the HELLP syndrome. AHUS should be suspected when hypertension is accompanied with MAHA, thrombocytopenia, renal function impairment and/or complications of abnormal vascular permeability. It should also be suspected when hypertension is severe and difficult to control with antihypertensive drugs. Correct diagnosis is essential for successful management of AHUS and prevention of its serious complications and even fatal outcomes.
References
- Isles CG, McLay A, Jones JM. Recovery in malignant hypertension presenting as acute renal failure. Q J Med. 1984; 53: 439-452.
- van den Born BJ, Honnebier UP, Koopmans RP, van Montfrans GA. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension. 2005; 45: 246-251.
- Amraoui F, Bos S, Vogt L, van den Born BJ. Long-term renal outcome in patients with malignant hypertension: a retrospective cohort study. BMC Nephrol. 2012; 13:71.
- Amraoui F, van der Hoeven NV, Van Valkengoed IG, Vogt L, van den Born BJ. Mortality and cardiovascular risk in patients with a history of malignant hypertension: a case-control study. J Clin Hypertens (Greenwich). 2014; 16:122-126.
- Fitzpatrick MM, Walters MD, Trompeter RS, Dillon MJ, Barratt TM. Atypical (non-diarrhea-associated) hemolytic-uremic syndrome in childhood. J Pediatr. 1993; 122: 532-537.
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaime F, Dragon-Durey MA, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013; 8: 554-562.
- Moser M, Goldman AG. Treatment of "accelerated" or malignant hypertension. I. Hypertensive encephalopathy. Med Times. 1966; 94:1008-1021.
- Tsai HM. A mechanistic approach to the diagnosis and management of atypical hemolytic uremic syndrome. Transfus Med Rev. 2014; 28:187-197.
- Tsai H-M. Thrombotic thrombocytopenic purpura, hemolytic uremic syndrome and related disorders. In Wintrobe's Clinical Hematology, 13/e, Chapter 48. JP Greer, J Foerster, G M Rodgers, F Paraskevas, B Glader, DA Arber & RT Means Jr (eds). Lippincott Williams & Wilkins, in 2013.
- Tsai HM. Does Anti complement Therapy Have a Role in the Management of Malignant Hypertension?. J Clin Hypertens (Greenwich). 2015; 18: 359-360.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol. 2014; 295323.
- Tsai HM, Kuo E. From Gestational hypertension and preeclampsia to atypical hemolytic uremic syndrome. Obstet Gynecol. 2016; 127: 907-910.
- Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011; 8: e1001013.
- Sinclair RA, Antonovych TT, Mostofi FK. Renal proliferative arteriopathies and associated glomerular changes: a light and electron microscopic study. Hum Pathol. 1976; 7: 565-588.
- Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010; 5: 1844-1859.