
Case Report
Austin J Nephrol Hypertens. 2016; 3(2): 1061.
A Case of Acetaminophen-Associated Acute Interstitial Nephritis
Nakamura H¹*, Makino M², Anayama M¹, Makino Y¹, Tamura K¹ and Nagasawa M¹
¹Department of Nephrology, Shinonoi General Hospital, Japan
²Department of Pathology, Shinonoi General Hospital, Japan
*Corresponding author: Hironori Nakamura, Department of Nephrology, Shinonoi General Hospital, 666-1 Ai Shinonoi, Nagano 388-8004, Japan
Received: September 28, 2016; Accepted: October 21, 2016; Published: October 24, 2016
Abstract
Few cases of acute interstitial nephritis associated with a therapeutic acetaminophen dose have been reported. A 33-year-old man developed an acute kidney injury after treatment with acetaminophen for fever. Rash, lymphadenopathy, petechiae, and arthralgia were not observed. Urinalysis indicated the presence of white blood cell casts, and computed tomography revealed bilateral kidney enlargement. Renal biopsy revealed massive infiltration of interstitial inflammatory cells and tubulitis. Following an extremely strong result for acetaminophen in drug lymphocyte stimulation tests, the patient was diagnosed with acetaminophen-associated acute interstitial nephritis. Therefore, acetaminophen should be considered an etiological agent of acute interstitial nephritis.
Keywords: Acute interstitial nephritis; Acetaminophen; Drug lymphocyte stimulation tests
Abbreviations
AIN: Acute Interstitial Nephritis; AKI: Acute Kidney Injury; u-NAG: urinary N-acetyl-β-D-glucosaminidase; β2MG: beta 2 Microglobulin; Ig: Immunoglobulin; PAS: Periodic Acid-Schiff; DLST: Drug Lymphocyte Stimulation Test; cpm: counts per minute; SI: Stimulation Index; TINU: Tubulointerstitial Nephritis and Uveitis
Introduction
Cases of renal failure related to drug-induced acute interstitial nephritis (AIN) are increasingly being reported in current medical practice. Although any drug can theoretically induce AIN, most cases of drug-induced AIN are attributed to antimicrobial and nonsteroidal anti-inflammatory drugs. Approximately 1%–2% of affected adult patients present with acetaminophen overdose-induced acute kidney injury (AKI) [1]. However, cases of AKI in patients treated with therapeutic doses of acetaminophen are rarely reported, particularly among adult populations [2,3]. We present here a rare case of AIN and AKI may be associated with a therapeutic dose of acetaminophen, which is considered relatively safe for renal health.
Case Presentation
In late February 2016, a 33-year-old man presented with fever and dry cough and was treated for 1 week with 500 mg of acetaminophen thrice per day, 50 mg of sitafloxacin twice per day, and 60 mg of loxoprofen sodium as needed. Seventeen days after the patient started to take medicines, the patient developed nausea and renal dysfunction and was referred and admitted to our hospital the next day for further study. At admission, his blood pressure was 142/82 mmHg and body temperature was 37.4°C. There was no evidence of rash, superficial lymphadenopathy, petechiae, or arthralgia.
Laboratory data including complete blood cell counts, common serum chemistry and immunological findings on admission are shown in Table1. Urinalysis revealed a gravity of 1.016, pH of 6.0, protein of 1+ (0.26 g/gCr), occult blood and glucose negativity, white blood cell casts, hematuria of 1–4, and 10–19 white blood cells per high-power field, with urinary N-acetyl-β-D-glucosaminidase (u-NAG) and beta 2 microglobulin (β2MG) levels of 27.3 IU/L and 19510 μg/L, respectively. Venous blood gas analysis indicated a pH of 7.37, pCO2 of 47 mmHg, and HCO3 of 26.7 mEq/L.
Laboratory data on admission
White blood cell
13100/μL
Blood urea nitrogen
28 mg/dL
Red blood cell
420×104 μL
Creatinine
2.8 mg/dL
Hemoglobin
12.3 g/dL
Uric acid
6.3 mg/dL
Hematocrit
36.70%
Platelet
34.8×104/μL
IgG
2025 mg/dL
Total protein
8.5 g/dL
IgA
398 mg/dL
Albumin
3.9 g/dL
IgM
113 mg/dL
Asparate transferase
29IU/L
IgE
62.6 IU/mL
Alanine transferase
44 IU/L
C3
148 mg/dL
Lactate dehydrogenase
207 IU/L
CH50
73.0 ch50/mL
Creatine phophokinase
78 IU/L
C-reactive protein
6.4 mg/dL
Total bilirubin
1.0 mg/dL
Anti-nuclear antibody
<40
Sodium
139 mEq/L
Anti-glomercular basement membrane antibody
<2.0 U/mL
Pottasium
4.4 mEq/L
PR3-ANCA
<1.0 U/mL
Chloride
101 mEq/L
MPO-ANCA
<1.0 U/mL
Calcium
9.8 mg/dL
Anti-SSA/ Ro antibody
<1.0 U/mL
Phosphorus
3.1 mg/dL
Anti-SSB/La antibody
<1.0 U/mL
Table 1: Laboratory data at admission. ANCA, anti-nuclear cytoplasmic antigen.
Computed tomography revealed a fatty liver and bilateral kidney enlargement (right, 124 mm×66 mm; left, 120 mm × 67 mm). No lymphadenopathy or interstitial lung lesions were detected. On day 2 after admission, a renal biopsy was performed to evaluate AKI and simultaneously manifesting liver dysfunction and hyperglobulinemia. Light microscopy findings revealed 11 glomeruli, one of which was global sclerosis. The other glomeruli were intact, with no apparent mesangial cell proliferation. As shown in (Figure 1A), hematoxylin–eosin staining (×100) and (Figure 1B), periodic acid-schiff (PAS; ×100) revealed massive, diffuse inflammatory cell infiltration in the interstitium and a glomerulus with no abnormality. Although a majority of inflammatory cells were lymphocytes and plasma cells, eosinophil were also noted. No detectable lesions in the small intralobular arteries were observed. PAS (×200) and periodic acid-methenamine-silver staining (×200) shown in (Figure 2A and 2B), respectively, revealed that tubulitis, i.e., massive infiltration of tubular epithelium by lymphocytes (large arrows) and macrophages (small arrows), was observed. Immunofluorescence analysis yielded negative results for Igs and non-specific positive results for C3.

Figure 1: Renal biopsy analysis reveals massive, diffuse inflammatory cell
infiltration in the interstitium. (A) Magnification, ×100, hematoxylin and eosin,
(B) Magnification, ×100, periodic acid-schiff.
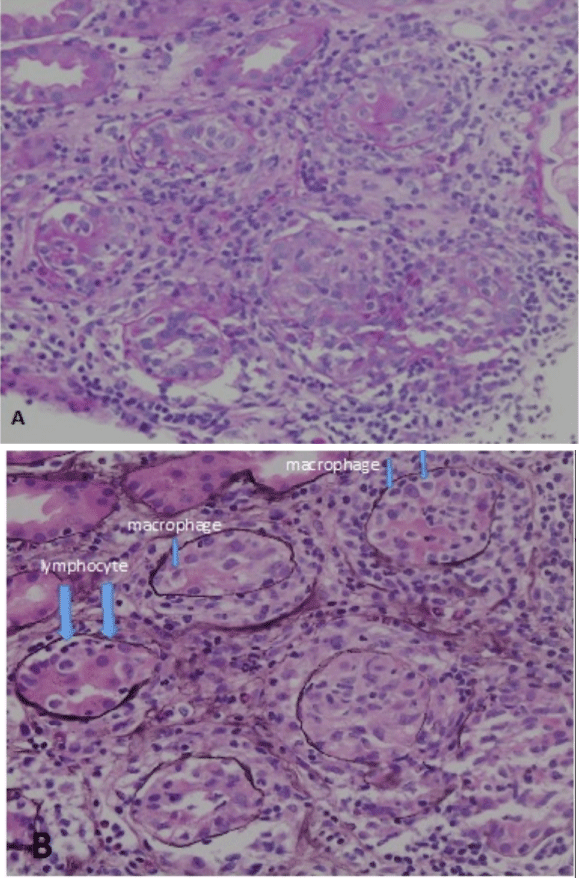
Figure 2: Tubulitis with some destructive tubules was observed. (A)
Magnification, ×200, periodic acid-Schiff, (B) Magnification: ×200, periodic
acid-methenamine-silver stain. Large arrows indicate lymphocyte and small
arrows indicate macrophage.
The patient was diagnosed with AIN according to pathological findings, which necessitated the differentiation of drug-induced AIN, Castleman disease, or Sjögren’s syndrome. Serological tests for antinuclear antibody, anti-neutrophil cytoplasmic antibody, anti-SSA/Ro antibody, or anti-SSB/Ro antibody were negative, thus eliminating the possibility of Sjögren’s syndrome. There were no elevated IgG4 or angiotensin-converting enzyme levels and M-protein expression were not detected. The patient did not present with ocular lesions. Drug lymphocyte stimulation test (DLST) results, obtained 10 days after admission, revealed strong positive findings for acetaminophen, with a value of 2572 counts per minute (cpm), normal range: below 180 cpm and a stimulation index (SI) of 1993%; in contrast, the results for loxoprofen sodium and sitafloxacin were considered negative at 231 cpm/179% and 136 cpm/105%, respectively. Based on these findings and the clinical treatment course, an association between AIN and a therapeutic acetaminophen dose was determined. A course of prednisolone (70 mg; 1 mg/kg) was started on day 7 to treat AIN. After 4 weeks, the patient’s renal dysfunction improved, as indicated by decreases in creatinine levels to 1.0 mg/dL (from 2.85 mg/dL) and in the u-NAG and urinary β2MG levels over time, as shown in (Figure 3).
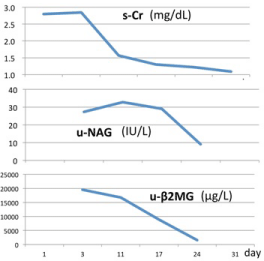
Figure 3: Changes in the levels of s-Cr, u-NAG, and u-β2MG over time.
Discussion
Here, we have described our experience with a patient who presented with fever, liver dysfunction, and renal dysfunction and described the process that led to a diagnosis of acetaminophenassociated AIN, based on both typical morphological findings from a renal biopsy as well as drug hypersensitivity. Praga, et al. reported that the etiologies of AIN could be classified as drug-induced, infectionrelated, idiopathic forms (including tubulointerstitial nephritis and uveitis [TINU] and anti-tubular basement membrane disease), and AIN associated with sarcoidosis and other systemic diseases such as systemic lupus erythematosus, Sjögren’s syndrome, or malignancy [4]. After excluding infection, systemic disease, sarcoidosis and malignancy in the present case, drug-induced AIN was strongly suspected and later confirmed by a positive DLST for acetaminophen.
AKI is very rarely associated with therapeutic doses of acetaminophen; to date, only a few cases involving adults [2,3] and a child [5] have been reported. Kato, et al. [3] reported the cases of two young adults with renal biopsy-proven acute tubular necrosis consequent to the use of therapeutic doses of acetaminophen. Although only one case exhibited a slightly positive DLST result for acetaminophen (186 cpm, SI unknown), Ki-67 staining revealed a strong proliferative activity among tubular cells recovering from necrosis. Ito, et al. [5] further reported the case of a 3-year-old girl who suffered from severe AKI with acute tubular necrosis and exhibited an SI for acetaminophen of 193%. The authors concluded that the girl suffered from biopsy-proven intrinsic AKI associated with a therapeutic dose of acetaminophen.
The pathological features of the present case, which included lymphocyte-dominant inflammatory cell infiltration of the interstitium, lack of IgG or C3 deposition to the tubular basement membrane, and strongly positive peripheral blood DLST for acetaminophen, support the notion that acetaminophen could cause AIN via cell-mediated mechanisms. We note that the DLST for the other two drugs administered to the patient were negative and observe that a positive test against only one drug in a treatment panel certainly facilitates identification of the relevant drug.
DLST, also known as lymphocyte transformation test, is based on the principle that T cells can proliferate in the presence of a specific antigen. The usefulness of DLST has been demonstrated in various diseases and with many different drugs. It is clinically used to determine drug hypersensitivity. Most results are given as SI: the proliferation is measured as 3H-thymidine uptake, cpm. This SI is calculated by proliferation (cpm) with drug/proliferation (cpm) without drug. Therefore, it was recommended that the test should not be performed in the acute stage as it may lead to false negative results, that is negative DLST cannot exclude a drug hypersensitivity. Some drugs, including vancomycin and paracetamol (also known as acetaminophen), as well as radio-contrast media, can slightly enhance the proliferation (SI 2–4) of peripheral blood mononuclear cells and may lead to a false-positive result [6]. However, the high SI for acetaminophen in the present case supports the role of this drug as the etiological agent. We must note that the results of a DLST may not be absolute, although a combination of this test and renal biopsy provides the most useful means by which a diagnosis of drug-related AIN can be confirmed and further evidence for the participation of cell-mediated immunity in the pathogenesis of drug-induced hypersensitivity nephritis can be obtained [7,8].
At therapeutic dosages, acetaminophen can induce renal toxicity in patients who are glutathione-depleted (e.g., chronic alcohol ingestion, starvation, fasting) or using drugs that stimulate P-450 microsomal oxidase enzymes (e.g., anticonvulsants) [9]. However, neither of these factors was observed in the present case. Although a therapeutic dose of acetaminophen has been reported to induce a slight but significant level of apoptosis in cultured tubular epithelial cells [10], we did not stain biopsy samples with a marker of apoptosis and therefore were unable to determine whether apoptosis had an effect on the disease pathology.
The therapeutic role of corticosteroids in AIN remains controversial, and the conclusions of the few large retrospective or prospective controlled studies that have been conducted are inconsistent. Although some studies have reported a more rapid and complete recovery of renal function with steroid administration, others have failed to confirm these results [11-14]. After starting prednisolone, the patient’s renal function improved gradually to normal levels over a 4-week period. However, we were unable to evaluate the efficacy of prednisolone on renal function in the present case.
Conclusion
This case of AIN and AKI may be associated with a therapeutic dose of acetaminophen, although rare, highlights the need for the awareness of the risk of acetaminophen-induced AIN.
References
- Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980; 10 2:291S-298S.
- Fruchter LL, Alexopoulou I, Lau KK. Acute interstitial nephritis with acetaminophen and alcohol intoxication. Ital J Pediatr. 2011; 15: 37: 17.
- Kato H, Fujigaki Y, Inoue R, Asakawa S, Shin S, Shima T, et al. Therapeutic dose of acetaminophen as a possible risk factor for acute kidney injury: learning from two healthy young adult cases. Intern Med. 2014; 53: 1531- 1534.
- Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010; 77: 956- 961.
- Ito T, Watanabe S, Tsuruga K, Aizawa T, Hirono K, Ito E, et al. Severe intrinsic acute kidney injury associated with therapeutic doses of acetaminophen. Pediatr Int. 2015; 57: e53-55.
- Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59: 809-820.
- Holland P, Mauer AM. Drug-induced in-vitro stimulation of peripheral lymphocytes. Lancet. 1964; 1: 1368-1369.
- Joh K, Aizawa S, Yamaguchi Y, Inomata I, Shibasaki T, Sakai O, et al. Druginduced hypersensitivity nephritis: lymphocyte stimulation testing and renal biopsy in 10 cases. Am J Nephrol. 1990; 10: 222-230.
- Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001; 31: 55-138.
- Lorz C, Justo P, Sanz A, Subirá D, Egido J, Ortiz A. Paracetamol-induced renal tubular injury: a role for ER stress. J Am Soc Nephrol. 2004; 15: 380- 389.
- Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001; 60:804- 817.
- González E, Gutiérrez E, Galeano C, Chevia C, de Sequera P, Bernis C, et al. Nefritis Intersticiales. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008; 73: 940-946.
- Clarkson MR, Giblin L, O’Connell FP,O’Kelly P, Walshe JJ, Conlon P, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004; 19: 2778-2783.
- Khaira A, Mendonca S. Steroids in acute interstitial nephritis. Kidney Int. 2008; 74: 971-972.