
Case Report
Austin J Nephrol Hypertens. 2019; 6(2): 1081.
A 31-Year-Old Lady with Post-Partum Systemic Lupus Erythematosus
Malaweera A1* and Huang LL2
1Department of Medicine, Eastern Health Clinical School, Monash University, Australia
2Department of Nephrology, Box Hill Hospital, Eastern Health, Australia
*Corresponding author: Malaweera A, Department of Medicine, Eastern Health Clinical School, Monash University, Australia
Received: May 02, 2019; Accepted: July 08, 2019; Published: July 15, 2019
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune systemic disease that is more common in women of child bearing age. Although the impact of pregnancy on SLE activity has been debated in the literature, majority of studies have noted increased SLE activity during pregnancy, causing peripartum complications like pre-eclampsia, pre-term labour and stillbirth. Our case is a 31-year-old woman who suffered pre-eclampsia during her pregnancy, presenting one month post-partum with haemolytic anaemia, decompensated cardiomyopathy with serositis and acute renal impairment. She was diagnosed with systemic lupus erythematosus with lupus nephritis and started on immunosuppressive therapy with hydroxychloroquine, corticosteroids and mycophenolate mofetil. This case highlights the importance of understanding the heterogeneous nature of SLE presentation with multiple organ involvement and the impact on SLE activity on pregnancy and its outcomes.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease affecting multiple organs. Its predominance in women, especially of child bearing age is characteristic for the disease. It is extremely heterogeneous with a wide range of clinical and serological manifestations and marked disease course in different individuals.
Although SLE can present commonly with constitutional symptoms like fatigue, weight loss and arthralgia, 25-50% patients can have serious organ involvement with lupus nephritis, pleural disease and myopericarditis. Additionally, SLE activity is associated with poor pregnancy and peri-partum outcomes with high risk of pre-eclampsia, pregnancy loss, pre-term birth, stillbirth and low birth weight1.
SLE is characterised by anaemia, hypocomplementemia and raised anti-nuclear antibody (ANA) and double stranded DNA antibody (dsDNA), the latter being diagnostic.
Today we present a challenging case of a 31-year-old presenting one month post-partum with anaemia, cardiomyopathy with a pericardial effusion, pleural effusions, arthralgia and acute renal failure.
Case Report
We present a 31-year-old Caucasian female (G1P1) who delivered twins through normal vaginal delivery 6 weeks prior to presenting to hospital with SLE. At 34 weeks, she was diagnosed as having preeclampsia with evidence of proteinuria, hypertension and deranged liver function tests. As a result, she underwent chemical induction and delivered twins via normal vaginal delivery without complications. She was hypertensive at >140/80 mmHg in the peri-partum period and was discharged with labetalol.
Her past medical history included Immune Thrombocytopenic Purpura (ITP) with previous steroid use and was currently in remission. She was in a family of four, with a history of SLE in both her brother and mother.
Two weeks after childbirth, she developed fatigue, shortness of breath on exertion and bilateral leg swelling and was diagnosed as having pleural effusions on chest x-ray. She received frusemide for this and her labetalol was changed to nifedipine. She was also found to have iron deficiency and anaemia with a haemoglobin of 68 g/L and was given an iron infusion. Other than mild vaginal bleeding post-delivery, she denied any other sources of bleeding.
Two weeks following this, she developed arthralgia in the small joints of the hands and a macular, blanching rash over her feet, knees, chest wall and hands but sparing the face.
She presented to a public hospital for further assessment and management of her anaemia and fluid overload. On clinical assessment, she had evidence of conjunctival pallor and a macular blanching rash over chest, hands and feet. She also had small joint synovitis with swelling and tenderness at proximal interphalangeal joints and pitting odema of the feet.
Her vital observations were stable throughout the admission with blood pressures of 120-130 mmHg systolic and 70-90 mmHg diastolic.
She had a normocytic anaemia of Hb 71 g/L, with a blood film consisting of mild anisocytosis with moderate normochromic normocytic anaemia with occasional tear drop and rare fragmented red cells. Her haemolysis screen revealed a positive coombs test, a low haptoglobin and high reticulocytes consistent with a haemolytic anaemia. Cross-matching studies also found that multiple positive antibodies making it difficult to find compatible blood for transfusion.
She also had acute kidney injury with a creatinine of 160 μmol/L, hyperkalaemia with a potassium of 6 mmol/L, metabolic acidosis with a bicarbonate of 16 mmol/L and a 24-hour urinary protein excretion of 0.64 g. Her kidney ultrasound revealed normal size kidneys without obstruction. Her urinalysis revealed 15 million/L erythrocytes without any cellular casts.
Additionally, she also had evidence of serositis. This was in the form of bi-basal (left>right) pleural effusions (Figures 1 & 2) and a moderate pericardial effusion with moderately-severe reduction in LV function on an Echocardiogram (Echo). She had a serum troponin 68 ng/L (‹14 ng/L) and a BNP was 3500 pmol/L (‹15.3 pmol/L) and her Electrocardiogram (ECG) revealed normal sinus rhythm.
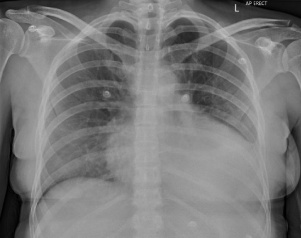
Figure 1: Chest Xray (CXR) showing a left sided pleural effusion.
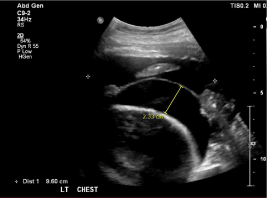
Figure 2: Ultrasound of Chest (USS) showing a left sided pleural effusion.
Her family history of SLE, pre-eclampsia during pregnancy and her current presentation with arthralgia, serositis, haemolytic anaemia and acute renal impairment, prompted an autoimmune screen. Her ANA was homogenously positive with a >2560 titre with reduced C3 and C4 of 0.33 g/L and 0.03 g/L, respectively. She also had a high dsDNA level of 4485 IU/ml confirming the diagnosis of SLE.
Due to the presence of severe haemolytic anaemia with multiple antibodies precluding a blood transfusion and shortness of breath making it difficult for the patient to lie supine, a renal biopsy was abandoned and she was empirically started on treatment.
She was given three pulses of intravenous methylprednisolone 500 mg followed by prednisolone 50 mg and hydroxychloroquine 200 mg daily. She was also started on mycophenolate mofetil (MMF) 1 g twice daily as immunosuppression for Lupus Nephritis. Two units of packed red cells were given for her anaemia once she was able to be cross-matched, while her cardiomyopathy was managed with Bisoprolol and Frusemide. The rest of her inpatient stay was unremarkable but showed an improvement in her renal function from a peak creatinine of 185 μmol/L to 93 μmol/L.
Discussion
Systemic lupus erythematosus is a multi-system autoimmune disease with a heterogeneous presentation. It occurs up to 10 times [1-3] more commonly in women, particularly women of child bearing age. It can present with a wide range of symptoms and signs include a rash, arthritis, serositis, nephritis and neuropsychological disturbance with psychosis and seizures. Constitutional symptoms like fatigue and fever seems to be the most common during presentation, rates being 50-90% [4]. Arthritis and arthralgia are also common, occurring in up to 90% of patients with a characteristic polyarticular, symmetrical distribution without erosion [5]. Skin manifestations include the classic malar butterfly rash occurring up to 50%, discoid skin lesions, urticaria and photosensitivity.
Concerningly, the morbidity and mortality in SLE is determined by its multi-organ involvement and dysfunction. Renal involvement in the most apparent and can occur in up to 40% of SLE patients [6]. The presentation and extent of disease is highly variable, ranging from asymptomatic haematuria/proteinuria to rapidly progressive glomerulonephritis. The pathogenesis is said to be due to deposition of immune complexes involving dsDNA antibodies on various sites in the kidney, namely the mesangium and the subendothelial region [7]. Normally, patients present with a raised creatinine, proteinuria and evidence of urinary sediment with cellular casts alongside elevation of dsDNA levels and hypocomplementemia. A kidney biopsy is indicated in most patients with lupus nephritis to establish a diagnosis but patients with ‹500 mg/day of proteinuria and bland urinary sediment may not require a kidney biopsy. After biopsy, lupus nephritis is divided into categories according to histopathological grading (Tables 1-3).
Day 1
Day 2
Day 3
Day 4
Day 5
Day 6
Day 7
Day 8
Day 9
Day 10
K (mmol/L)
6.0
5.7
6.1
5.1
4.7
4.1
4.2
5.0
4.5
3.8
Urea (mmol/L)
18
20
22.5
28.4
25.6
21.3
15.1
14.1
14.3
14.4
Creatinine (mmol/L)
160
179
185
168
139
137
95
113
102
93
eGFR
(mL/min/1.73m2)
36
32
30
34
43
44
68
55
63
70
Hb (g/L)
71
72
67
66
57
55
65
94
92
89
ACR
(mg/mmol)
136.5
PCR
(g/mmol)
0.180
K: Potassium; U: Urea; Creat: Ccreatinine; eGFR: estimated glomerular filtration rate (CKD-EPI); Hb: Haemoglobin; ACR: Albumin to Creatinine Ratio; PCR: Protein to Creatinine Ratio.
Table 1: Laboratory results during hospitalisation.
Autoimmune screen
Patient Value
Normal Value
ANA
Positive. Homogenous >2560 titre
Negative
dsDNA (IU/ml)
4485
< 100 IU/ml
C3 level (g/L)
0.33
0.9-1.8 g/L
C4 level (g/L)
0.03
0.1-0.4 g/L
ANCA (units/ml)
<5
< 5 units/ml
RF (kunits/L)
<10
< 10 kunits/L
ENA
Negative
Negative
Anti PLA2R (RU/ml)
<2
< 2 RU/ml
ESR (mm/hr)
117
10-15 mm/hr
CRP (mg/L)
29
< 5 mg/L
Lupus anticoagulant
Negative
Negative
Reticulocytes %
6.5 %
0.5-2%
Haptoglobin (g/L)
<0.10 g/L
0.3-2g/L
Bilirubin (μmol/L)
2
22 μmol/L
Direct coombs test
Positive
Negative
ANA: Antinuclear Antigen; dsDNA: Double stranded Deoxyribonucleic acid; C3: Complement 3 level; C4: Complement 4 level; ANCA: Anti Neutrophil Cytoplasmic Antibody; RF: Rheumatoid Factor; ENA: Extractable Nuclear Antigen; Anti PLA2R: Anti Phospholipase A2 antibodies; ESR: Erythrocyte Sedimentation Rate; CRP: C - Reactive Protein
Table 2: Autoimmune, haemolytic and thrombophilia panel showing evidence of SLE and haemolytic anaemia.
Class
Type of abnormality
Management
I
Minimal mesangial Lupus Nephritis
Monitoring
II
Mesangial proliferate Lupus Nephritis
Generally, a RAAS inhibitor but can consider immunosuppressive therapy for proteinuria >1g/24 hours
III
Focal Lupus Nephritis (involving <50% glomeruli)
III A: active lesions
III A/C: active and chronic lesions
III C: chronic lesions
Induction and Maintenance therapy with steroids and MMF/Cyclophosphamide
IV
Diffuse Lupus Nephritis (involving >50% glomeruli)
IV A: active lesions
IV A/C: active and chronic lesions
IV C: chronic lesions
Induction and Maintenance therapy with steroids and MMF/Cyclophosphamide
V
Membranous Lupus Nephritis
Consider steroids for proteinuria >3g/24 hours
VI
Advanced sclerosing Lupus Nephritis
Preparation of Renal replacement therapy
Table 3: Classes of lupus nephritis according to renal biopsy.
Pulmonary involvement is also common in SLE, occurring in up to 90% of patients on autopsy [8]. It manifests as pleurisy and exudative pleural effusions with a high LDH [8]. Cardiac disease in SLE patients can involve many areas of the heart, notably pericarditis +/- effusion being the most common in up to 25 % of the patients [9]. Other cardiac manifestations include myocarditis and coronary artery disease. Gastrointestinal involvement occurs in up to 40% patients and can result in oesophagitis, pseudo-obstruction, hepatitis, pancreatitis and mesenteric ischemia [10].
Haematological manifestations are common in SLE and span from anaemia, thrombocytopenia and lymphopenia. The causes of anaemia in SLE patients could be multifactorial with anaemia of chronic disease due to chronic inflammation being the most common. Other causes include iron deficiency, aplastic anaemia and autoimmune haemolytic anaemia. Autoimmune haemolytic anaemia has a prevalence of about 10% in SLE patients [11,12] and is associated with other significant organ dysfunction including renal, cardiac and pleural disease. This type of anaemia presents with increased reticulocyte count, raised LDH, raised indirect bilirubin, low haptoglobin and a positive coombs test. Interestingly, a phenomenon called Evans syndrome exists where there is occurrence of two or more haematological immune cytopenias, with ITP and AIHA combination being the most common [11,12]. In addition, in 50% of these patients, this could be a manifestation of SLE and present before the onset of SLE [11-13]. A study on 26 patients in 2006 looking at treatment of severe autoimmune haemolytic anaemia in SLE patients found that corticosteroids were the best first line of treatment for the anaemia [14].
Neuropsychiatric manifestations cannot only be frequent in up to 90% patients with SLE but can be on a wide spectrum from peripheral neuropathies, psychosis, seizures to cognitive dysfunction [15].
The relationship between and pregnancy and SLE is interesting and has been thoroughly reviewed in literature. The effect of SLE on fertility is debatable. Although some studies have suggested a reduction in fertility amongst SLE patients due to age, less ovarian reserve with cyclophosphamide, disease activity and concurrent antiphospholipid syndrome [16-18], others have not observed differences in fertility due to good disease control prior to conception and less use of cyclophosphamide [16-19].
Another concept debated in the literature is SLE activity in pregnancy. Whilst some studies found no significant increase in SLE activity between matched pregnant and non-pregnant SLE patients [19], majority of the studies found high SLE activity in pregnant patients. These studies collectively demonstrated a 2-3-fold increase in SLE activity during pregnancy [2,16-19], most being mildmoderate disease activity. The pathophysiology of this could be due to T cell activity. A healthy pregnancy leads to a shift from TH1 to TH2 mediated immune response, enabling the mothers’ immune tolerance to the foetus. SLE is considered a TH2 mediated disease, therefore may worsen during pregnancy [20]. Risk factors for this seem to be active disease before conception and multiple flares in the years prior to conception [2]. It is observed that SLE flares can occur anytime during pregnancy and importantly, several months after delivery [2].
However, literature on SLE and negative pregnancy outcomes has been consistent. SLE has been associated with an increased risk of preeclampsia, pregnancy loss, pre-term labour, stillbirth and low birth weight. Pre-eclampsia rates range from 13-35% [2] in SLE patients compared to general population with risk factors being first pregnancy, nulliparity, renal disease, low complement levels, hypertension and dsSDNA positivity [2]. Patients with lupus nephritis have the highest risk with some studies showing rates up to 66% [2]. Pregnancy loss and stillbirth are also higher in SLE, with a rate of up to 20% with risk factors being SLE activity and antiphospholipid syndrome [2]. Preterm birth is notably increased in SLE with rates up to 30% with risk factors being hypertension, SLE activity and higher prednisolone doses [2]. Finally, low birthweights less than 10th percentile are also found in 10% of live-births in women with SLE [2,21].
Whilst the diagnosis of SLE was not obvious during our patients’ episode of pre-eclampsia, presence of pre-eclampsia is a significant marker of her subacute presentation of SLE. Although pregnancy is not contra-indicated in SLE patients, careful counselling and monitoring of patient through conception, pregnancy and post-partum period is important. Should our patient become pregnant again, remission of SLE activity 6 months prior to conception and careful co-ordination of the high-risk pregnancy with a multidisciplinary team approach is key [2].
The connection between antiphospholipid syndrome and SLE is also important. About 40% SLE patients have positive antiphospholipid antibodies although development of secondary antiphospholipid syndrome in SLE is slightly less common [22]. Antiphospholipid syndrome is characterized by arterial and venous thrombosis and multiple pregnancy complications including preeclampsia, fetal death and placental insufficiency [23].
The specific management of SLE largely depends on the predominant organ system that is involved but a large evidence base has suggested that all patients should be on hydroxychloroquine unless contraindicated [24-26]. A large systematic review revealed that hydroxychloroquine has multiple benefits including reducing flares, protecting in against organ damage, reducing thrombosis and increasing long term survival [24]. Other studies have also revealed less skin and integument damage with the use of hydroxychloroquine [24-26].
Lupus nephritis treatment depends usually on the features found at renal biopsy (Table 1) [27,28]. Generally, aggressive immunosuppressive treatment is reserved for classes III and IV with active lesions.
Although the effects of treatment on all-cause mortality was difficult to elicit, KDIGO guidelines for lupus nephritis suggested that induction therapy should consist of corticosteroids and another agent. Whilst cyclophosphamide and MMF both lead to similar rates of disease remission and reduction of time to doubling of creatinine, MMF had lower risk of ovarian failure. Therefore, MMF is the preferred treatment in women of childbearing age, like in our case [29]. For maintenance therapy, evidence shows MMF being superior compared to other agents like azathioprine, which increases rates of renal relapse and leukopenia [29].
After appropriate treatment, patients usually go into remission. Rarely, some patients do not require further treatment after the initial presentation but most lead a relapsing and remitting disease course. The prognosis of SLE varies significantly between individuals and depends on various factors with poor prognostic factors being the presence of renal disease, co-existing hypertension, male sex, black race and presence of antiphospholipid syndrome. Although the overall survival has improved in the last decade owing to increased disease recognition and prompt treatment, SLE still has higher mortality rates up to 5 times more than the general population [30].
In summary, the presentation of SLE in the post-partum period is important to note in a young patient with strong family history of autoimmune disease. It is also important to understand that SLE presents heterogeneously with multiple organ involvement with varying severity. Therefore, prompt diagnosis with thorough clinical assessment, laboratory autoimmune panel testing and management of organ disease with an appropriate level of immunosuppression is crucial, to improve the overall prognosis. Finally, it is important to understand the close interaction between pregnancy and SLE with its multiple negative effects on both maternal and fetal morbidity and mortality. The overall management of an individual with SLE should be carried out in a multidisciplinary team involving physicians of different specialties, nursing and allied health staff and psychologists.
Abstract
References
- Iozza I, Cianci S, Natale A, Garofalo G, Giacobbe AM, Giorgio E, et al. Update on systemic lupus erythematosus pregnancy. Journal of Prenatal Medicine [Online]. 2010; 4: 67-73.
- Clowse M. Lupus activity in Pregnancy. Rheumatic Disease clinics of North America. 2007; 33: 237-v.
- D’Cruz D, Munther A, Khamashta FRCP, Graham RV, Hughes FRCP. Systemic Lupus Erythromatosus. The Lancet. 2007; 369: 587-596.
- Tench C, McCurdie I, White PD, D’Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology. 2000; 39: 1249- 1254.
- Grossmann J. Lupus Arthritis. Best Practice & Research: Clinical Rheumatology. 2009; 23: 495-506.
- Hoover P, Karen H. Costenbader. Insights into the Epidemiology and Management of Lupus Nephritis from the U.S Rheumatologist’s Perspective. Kidney international. 2016; 90: 487-492.
- Yung S, Tak Mao Chan. Mechanisms of Kidney Injury in Lupus Nephritis- the Role of Anti-dsDNA Antibodies. Frontiers in Immunology. 2015; 6: 475.
- Keane M, Joseph P, Lynch III. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000; 55: 155-166.
- Miner J, Kim AH. Cardiac Manifestations of systemic lupus erythematosus. Rheumatic Disease Clinics of North America 2014; 40: 51-60.
- Xin-Ping T, Xuan Zhang. Gastrointestinal involvement in systemic lupus erythematosus: Insight into pathogenesis, diagnosis and treatment. World Journal of Gastroenterology 2010; 16: 2971-2977.
- Giannouli S, M Voulgarelis, PD Ziakas, AG Tzioufas. Anaemia in systemic lupus erythematosus: from pathophysiology to clinical assessment. Annals of the Rheumatic diseases. 2006; 65: 144-148.
- Jeffries M, Hamadeh F, Aberle T, Glenn S, Kamen DL, Kelly JA. Haemolytic anaemia in a multi-ethnic cohort of lupus patients: a clinical and serological perspective. Lupus. 2008; 17: 739-743.
- Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, Cirasino L. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009; 114: 3167-3172.
- Gomard-Mennesson E, M Ruivard, M Koenig, A Woods, N Magy, J Ninet. Treatment of isolated severe immune haemolytic anaemia associated with systemic lupus erythematosus: 26 cases. Lupus. 2006; 15: 222-231.
- Gulinello M Jing Wen, MD, Chaim Putterman MD. Neuropsychiatric symptoms in Lupus. Psychiatric Annals. 2012; 42: 322-328.
- Jones A, Ian Giles. Fertility and pregnancy in systemic lupus erythematosus. Indian Journal of Rheumatology. 2016; 11: 128-134.
- Bermas B, Lisa R, Sammaritano. Fertility and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Fertility Research and Practice. 2015; 1: 13.
- Meehan R, Dorsey JK. Pregnancy among patients with systemic lupus erythematosus receiving immunosuppressive therapy. Journal of Rheumatology. 1987; 14: 252-258.
- Lima F, Buchanan NM, Khamashta MA, Kerslake S, Hughes GR. Obstetric outcome in systemic lupus erythematosus. Seminars in Arthritis and Rheumatism. 1995; 25: 184-192.
- Doria A, Maurizio Cutolo, Anna Ghirardello, Margherita Zen, Danilo Villalta, Angela Tincani. Effect of pregnancy on serum cytokines in SLE patients. Arthritis Research & Therapy. 2012; 14: R66.
- Poon MY Tan, G Yerlikaya, A Syngelaki, KH Nicolaides. Birth weights in live births and stillbirths. Ultrasound in Obstetrics & Gynaecology. 2016; 48: 602- 606.
- Unlu O, Stephane Zuily, Doruk Erkan. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. European Journal of Rheumatology. 2016; 3: 75-84.
- Abrahams V, Lawrence W, Chamley, Jane E. Salmon. Antiphospholipid Syndrome and Pregnancy: Pathogenesis to Translation. Arthritis & Rheumatology. 2017; 69: 1710-1721.
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Annals of Rheumatological diseases. 2010; 69: 20-28.
- Akhavan, P, Su J, Lou W, Gladman DD, Urowitz MB, Fortin PR. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. Journal of Rheumatology. 2013; 40: 831-841.
- Pons-Estel G, Alarcón GS, González LA, Zhang J, Vilá LM, Reveille JD. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis care and Research. 2010; 62: 393-400.
- Hahn B, Maureen A, Mcmahon, Alan Wilkinson, W. Dean Wallace, David I Daikh. American College of Rheumatology Guidelines for Screening, Treatment, and Management of Lupus Nephritis. American College of Rheumatology. 2012; 64: 797-808.
- Wilhelmus S, Ingeborg M Bajema, George K Bertsias, Dimitrios T Boumpas, Caroline Gordon, Liz Lightstone. Lupus Nephritis management guidelines compared. Nephrology Dialysis Transplantation. 2016; 31: 904-913.
- KDIGO Glomerulonephritis Guideline update- Evidence Summary. Lupus Nephritis.
- Singh, R, Yen EY. SLE mortality remains disproportionately high, despite improvements over the last decade. Lupus. 2018; 27: 1577-1581.