
Research Article
Austin J Nephrol Hypertens. 2021; 8(2): 1096.
Erythropoietin Resistant Anaemia among Haemodialysis Patients with Malnutrition Inflammation Complex Syndrome in Dar es Salaam, Tanzania
Bramania PK¹*, Ruggajo PJ¹ and Furia FF²
1Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Tanzania
2Department of Pediatrics and Child Health, Muhimbili University of Health and Allied Sciences, Tanzania
*Corresponding author: Bramania PK, Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, P.O. Box 20577, Dar es Salaam, Tanzania
Received: August 02, 2021; Accepted: September 02, 2021; Published: September 09, 2021
Abstract
Background: Erythropoietin-resistant anaemia in hemodialysis patients is accompanied by poor outcomes. Malnutrition and inflammation impair effective erythropoiesis through various mechanisms and may cause erythropoietin resistance. In this study, we aimed to determine the effect of malnutrition inflammatory complex on erythropoietin resistance among patients on maintenance hemodialysis at Muhimbili National Hospital in Dar es Salaam, Tanzania.
Materials and Methods: This was a hospital-based retrospective cross-sectional study involving 85 patients on maintenance hemodialysis. Participants’ information was collected and recorded in data collection tools; this information included clinical and physical information (body weight and height) and laboratory tests (complete blood count, serum albumin, C-reactive protein, transferrin, total iron, ferritin, and urea). Bodyweight and height were measured and body mass index calculated. Erythropoietin Resistance Index (ERI) was obtained as the weight-adjusted average weekly erythropoietin dose divided by hemoglobin level, while Malnutrition Inflammation Score (MIS) was used to determine Malnutrition Inflammation Complex Syndrome (MICS).
Results: Eighty-five participants were recruited for this study, of which 76.5% were males and the mean age was 54.1 ± 13.2 years. MICS was noted in 50.6% participants. The overall mean weight-adjusted ERI of the study population was 20.6 ± 7.7 units/kg per g/dl. Patients with inflammation, MICS, and on long-term hemodialysis had significantly higher mean ERI. On multivariate analysis, ERI significantly correlated with MIS (p <0.01) in a dose-dependent manner.
Conclusion: In hemodialysis patients, anaemia resistant to Erythropoietin therapy is linked to malnutrition inflammation complex syndrome. MICS needs to be appropriately treated to achieve target hemoglobin levels.
Keywords: Erythropoietin resistance index; Malnutrition inflammation complex syndrome
Abbreviations
ANOVA: Analysis of Variance; BMI: Body Mass Index; CBC: Complete Blood Count; CKD: Chronic Kidney Disease; CI: Confidence Interval; CRP: C-reactive Protein; EPO: Erythropoietin; ERI: Erythropoietin Resistance Index; ESA: Erythropoietin Stimulating Agents; HD: Hemodialysis; HIV: Human Immunodeficiency Virus; Il-6: Interleukin-6; KDIGO: Kidney Disease Improving Global Outcomes; MIS: Malnutrition Inflammation Score; MICS: Malnutrition Inflammation Complex Syndrome; MNH: Muhimbili National Hospital; MUHAS: Muhimbili University of Health and Allied Sciences; OR: Odds Ratio; SPSS: Statistical Package of Social Sciences; TSAT: Transferrin Saturation; URR: Urea Reduction Ratio
Introduction
Erythropoietin (EPO) deficiency is the major contributor of anaemia among patients with Chronic Kidney Disease (CKD) requiring regular EPO injection as the main treatment modality [1]. A high prevalence of anaemia (69%) was noted among patients on Haemodialysis (HD) in Tanzania, the majority of which (84%) had iron deficiency anaemia [2]. EPO hypo-responsiveness in patients on dialysis portends a poor prognosis and is attributed to several factors: iron deficiency, ongoing blood loss, malnutrition, inflammation, comorbidities, and severe hyperparathyroidism [3-5]. It can be categorized as an initial failure of the rise of hemoglobin level despite an appropriate weight-adjusted dose of EPO or an acquired hypo-responsiveness defined as requiring two increments in EPO stimulating agents (ESA) up to 50% beyond the stable dose [3,4].
ERI has been introduced to describe EPO hypo-responsiveness adjusted based on the severity of anaemia [3,4]. ERI is defined as the weekly EPO dose per body weight divided by hemoglobin level [3-5].
Haemodialysis patients with undernutrition were noted to have higher ERI [5]. Poor dietary intake and micronutrient deficiency have a profound effect on effective erythropoiesis [3,6,7]. MICS, which is evaluated by using the malnutrition inflammation score, has been closely correlated with poor response to EPO therapy similar to other markers of inflammation including Interleukin-6 (IL-6), C-Reactive Protein (CRP), and ferritin [8-11]. Resistance to EPO results in higher doses to maintain target haemoglobin levels. This increases the cost of treatment and exposes patients to unwanted adverse effects from EPO therapy [12]. Improving the quality of dialysis therapy reduces inflammation and will subsequently minimize EPO requirements [3,13].
Limited data on EPO response among HD patients in the region led to designing this study, which was aimed at determining the association between malnutrition inflammation complex and EPO response (expressed as ERI) among patients on maintenance hemodialysis at Muhimbili National Hospital (MNH) in Dar es Salaam, Tanzania.
Materials and Methods
Study design, setting, population, and sample size
This was a retrospective cross-sectional analysis of a study conducted in two hemodialysis centers of Muhimbili National Hospital located at Upanga and Mloganzila in Dar es Salaam, Tanzania. Data were collected from September to November 2019.
Patients aged 18 years and above who were on maintenance hemodialysis therapy for at least 3 months were eligible for this study. Additional information regarding EPO use and laboratory tests for iron parameters (iron, ferritin, transferrin saturation) were collected for 160 participants who were part of the study that investigated the prevalence and correlates of MICS [14].
Patients who had received a recent blood transfusion, those with febrile illness, vascular site infection, those not on EPO therapy at the time of the study, those with missing records, and those with chronic diseases (chronic viral infections, known chronic autoimmune diseases) were excluded from the final analysis.
Of the 160 participants’ assessed for MICS, 75 were excluded due to the following reasons: 6 patients had received a blood transfusion within the preceding three months, 3 patients had febrile illness in the preceding one week, 1 patient had evidence of vascular access site infection, 6 patients were not receiving EPO at the time of the study, 34 patients had some missing records of administered EPO, and or some iron profile parameters. Furthermore, 25 patients with known chronic inflammatory conditions (15 had Human Immunodeficiency Virus (HIV) infection, 9 patients had Hepatitis B infection, and one patient had Systemic lupus erythematosus) were excluded leaving 85 patients for eventual analysis of this current study.
Data collection methods
Participants’ biodata, clinical and, hemodialysis-related information was collected through a face-to-face interview by the principal investigator and recorded using a data collection tool. The total dose of EPO injections administered over the preceding month was obtained from their hemodialysis records. Participant’s dry weight three months before the date of the interview was obtained from the individual dialysis record files. Dry weight change within a period of the past three months, dietary intake, gastrointestinal symptoms, nutritional-related functional impairment, and comorbidity status were enquired and graded based on the MIS. The severity of loss of fat stores was assessed at eyes, triceps, biceps, and chest. Muscle wasting (assessed at the temple, clavicle, scapula, ribs, quadriceps, knee, interosseous), body size (measured by Body Mass Index (BMI)), serum albumin, and transferrin were graded based on the MIS. The MIS is a comprehensive scoring system that encompasses ten parameters, each graded from 0 (normal) to 3 (abnormal). MIS correlates with morbidity and mortality in HD patients and is used to assess MICS defined by an aggregate MIS of 6 or above [8].
In haemodialysis units at MNH, the recombinant EPO injections containing 4,000 international units are given intravenously to patients on the days they receive hemodialysis therapy. We searched for participants’ dialysis records over the preceding 1 month before the date of data collection and calculated the total dose of EPO received in international units. The average weekly EPO dose was then determined by dividing the total monthly dose by four. Weightadjusted EPO dose was based on the dry weight measured at the time of data collection.
Anthropometric measurements and laboratory tests
Participant’s dry weight (post-dialysis weight) was determined using a standard weighing scale. Height was determined using a stadiometer. The length from the top of the head to the heel of the feet was taken in lieu of height for those who could not stand upright on the stadiometer. Underweight was defined as having a BMI below 18.5 kg/m².
Participants’ blood specimens (10ml) were drawn and tested for Complete Blood Count (CBC), serum albumin, transferrin, iron, ferritin, CRP, pre-and post-dialysis urea. CBC was analyzed using CELL DYN 3700 machine whereas the stated biochemical tests were analyzed in the ARCHITECT PLUS machine. CRP was analyzed by fluorescence immunoassay-based Finecare Rapid Quantitative test using COBAS INTEGRA 400 machine.
Study variables
The main output variable in this study was the weight-adjusted ERI, which is a measure of EPO responsiveness. It was assessed using the formula ERI = Total weekly EPO dose (units) ÷ hemoglobin (g/dl) ÷ post-dialysis weight (kg) [3-5]. MICS was categorized as mild (MIS of 6 to 10) and moderate to severe (MIS of 11 to 30) [14]. Hypoalbuminemia was defined as having serum albumin <4g/dl as per the MIS [8]. A CRP level above 5mg/l was defined as having Inflammation [14]. The Urea Reduction Ratio (URR) was assessed using the formula (Pre-dialysis Urea – Post-dialysis Urea) ÷ Pre-dialysis Urea x 100%. Adequate hemodialysis was regarded as having a URR of 65% or above [14]. Anaemia was diagnosed by a hemoglobin level below 12 g/dl in females and 13 g/dl in males based on the KDIGO Clinical practice guideline for anaemia in CKD [4]. Iron deficiency was defined as having Transferrin Saturation (TSAT) of <30% and/or a serum ferritin <500ng/ml [4]. The transferrin saturation in percentage was calculated using the formula TSAT (%) = Serum Iron (in μmol/l) ÷ Serum transferrin (in mg/dl) x 398 [15].
Data analysis
Data collection tools were assessed for completeness, then the data were entered electronically into the Statistical Package of Social Sciences (SPSS) software version 20. Categorical variables were summarized as proportions while means and medians were used for continuous variables. The mean ERI was compared across different categorical variables using the Analysis of Variance (ANOVA) test. Univariate and multivariate linear regression analysis was used to determine factors associated with weight-adjusted ERI. The four quartiles of ERI were determined and then the crude (unadjusted) odds ratio of the highest quartile versus the three lower quartiles was computed using the binary logistical regression analysis. Multivariate binary logistic regression analysis was performed to determine the adjusted odds of the highest quartile of ERI. Variables having a univariate p <0.2 were included in the multivariate analysis. A p-value of <0.05 was regarded as statistically significant.
Ethical considerations
The Institutional Review Board of the Muhimbili University of Health and Allied Sciences (MUHAS) Ethical Committee approved this study and the permission for this study was provided by the hospital administration. All participants provided written informed consent before recruitment.
Results
Demographic and clinical characteristics of the study population
Among the 85 participants on maintenance HD, 65 (76.5%) were male. The mean age of the participants was 54.1 ± 13.2 years, and the median duration on HD was 19 (9 to 30.5) months. Most of them, 78 (91.8%) were receiving standard three times per week HD services, and over a quarter (27.1%) were found to receive inadequate HD therapy.
Almost half (52.9%) of the participants had diabetes mellitus and virtually all (96.5%) had Hypertension. Anaemia was prevalent in 90% of female, and almost all (98.5%) male participants. The overall mean hemoglobin level of the study population was 9.28 ± 1.83 g/dl. Iron deficiency was present in 67 (78.8%) of the participants (Table 1).
Characteristics
n (%) or mean ± SD
Gender (Male)
65 (76.5%)
Age (years)
54.1 ± 13.2
Diabetes Mellitus
45 (52.9%)
Hypertension
82 (96.5%)
Duration on HD (months)
24 ± 20.5
HD frequency (Thrice/week)
78 (91.8%)
Vascular access (AV fistula)
43 (50.6%)
Underweight
17 (20.0%)
BMI (kg/m2)
22.3 ± 4.0
Hypoalbuminemia
65 (76.5%)
Serum albumin (g/dl)
3.6 ± 0.5
Anaemia in Females, n=20
18 (90.0%)
Hemoglobin (g/dl)
9.1 ± 1.9
Anaemia in Males, n=65
64 (98.5%)
Hemoglobin (g/dl)
9.3 ± 1.8
Iron Deficiency
67 (78.8%)
Transferrin Saturation (%)
21.2 ± 13.9
Inadequate Hemodialysis
23 (27.1%)
Urea Reduction Ratio, URR (%)
69.3 ± 12.0
Inflammation
72 (84.7%)
CRP (mg/l)
15 (7.6 to 33.8)*
Mild MICS
18 (21.2%)
Moderate to Severe MICS
25 (29.4%)
*Median (Interquartile range).
Table 1: Demographic and clinical characteristics of participants, N=85.
Nutritional and inflammation status of the study population
Of the 85 patients on HD, 17 (20%) were underweight. Serum albumin (a surrogate biomarker of nutritional status) was below the recommended level (4g/dl) in almost three-fourths (76.5%) of the participants. Seventy-two participants (84.7%) had inflammation (defined as serum CRP levels above 5mg/l). MICS was present in 43 (50.6%) participants (mild in 21.2% and moderate to severe in 29.4%) (Table 1).
Anaemia, Erythropoietin use and responsiveness measures
Amongst the 85 hemodialysis, patients receiving maintenance EPO therapy, 73 (85.9%) were receiving EPO three times per week. Most of them, 76 (89.4%) had a hemoglobin level below the KDIGO target of 11.5g/dl [4]. The overall mean weekly EPO dose was 182 ± 50 units/kg. In the month preceding the study, 66 patients were receiving at least 150units/kg of EPO per week, amongst them, 61 (92.4%) had hemoglobin below the recommended target of 11.5g/dl [4]. EPO responsiveness was assessed using the ERI. The overall mean weight-adjusted ERI of the study population was 20.6 ± 7.7 units/kg per g/dl.
Association between ERI, MIS, and other clinical laboratory parameters
MIS was significantly greater across the quartiles of ERI (Figure 1). In univariate linear regression analysis; HD duration, CRP, and MIS correlated with the ERI. In multivariate linear regression analysis, age of participants and MIS significantly correlated with the ERI (Table 2).
Parameters
Univariate B (95% CI)
p-value
Multivariate B (95%CI)
p-value
Age
-0.121 (-0.247 to 0.004)
0.058
-0.158 (-0.261 to -0.055)
0.003
HD Duration
0.122 (0.044 to 0.200)
0.002
0.029 (-0.043 to 0.101)
0.427
TSAT
-0.085 (-0.205 to 0.035)
0.163
-0.017 (-0.118 to 0.083)
0.732
CRP
0.071 (0.023 to 0.119)
0.004
-0.013 (-0.063 to 0.037)
0.601
URR
0.058 (-0.083 to 0.198)
0.416
0.036 (-0.080 to 0.151)
0.539
MIS
0.800 (0.551 to 1.048)
<0.001
0.839 (0.511 to 1.166)
<0.001
B: Unstandardized regression coefficient; CI: Confidence Interval.
Table 2: Linear regression analysis to show the parameters associated with weight-adjusted Erythropoietin resistance index among hemodialysis patients, N=85.
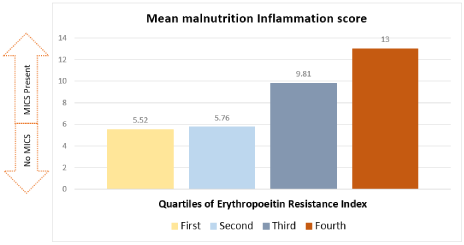
Figure 1: Mean malnutrition inflammation scores with respect to the quartiles of Erythropoietin resistance index among hemodialysis patients.
The mean ERI was significantly higher among patients with MICS (24.3 versus 16.8 units/kg per g/dl, p <0.01). Also patients receiving HD therapy for longer than 4 years had significantly higher mean ERI. Similarly, patients with inflammation had significantly greater mean ERI (Table 3).
Characteristics
Mean ERI Units/kg/(g/dl)
p-value
Unadjusted OR (95%CI)
p-value
Adjusted OR (95%CI)
p-value
Age groups
>60 years
18.7 ± 6.3
0.06
Ref
0.4
-
-
18-59 years
21.9 ± 8.5
1.56 (0.55-4.37)
Gender
Male
20.0 ± 6.7
0.22
Ref
0.23
-
-
Female
22.5 ± 10.6
1.96 (0.66-5.85)
Duration of HD
< 4 years
19.8 ± 6.9
0.01
Ref
0.34
-
-
> 4 years
26.1 ± 10.7
1.92 (0.50-7.34)
Frequency of HD
Thrice per week
20.5 ± 7.1
0.68
Ref
0.81
-
-
Twice per week
21.8 ± 13.8
1.24 (0.22-6.93)
Dialysis Adequacy
Adequate
20.7 ± 8.4
0.92
Ref
0.35
-
-
Inadequate
20.5 ± 6.0
0.56 (0.17-1.88)
Vascular access
AV Fistula
20.1 ± 7.6
0.55
Ref
0.19
Ref
0.16
Dialysis Catheter
21.1 ± 8.0
1.96 (0.72-5.38)
2.19 (0.74-6.50)
Iron deficiency
Absent
20.2 ± 7.5
0.79
Ref
0.38
-
-
Present
20.7 ± 7.9
1.84 (0.48-7.1)
Inflammation
Absent
15.1 ± 5.1
<0.01
Ref
0.15
Ref
0.87
Present
21.6 ± 7.7
4.62 (0.56-37.8)
1.22 (0.11-13.2)
MICS
Absent
16.8 ± 5.2
<0.01
Ref
0.003
Ref
0.009
Present
24.3 ± 8.1
6.21 (1.88-20.6)
6.22 (1.59-24.4)
OR: Odds Ratio; HD: Hemodialysis; MICS: Malnutrition Inflammation Complex Syndrome.
Table 3: Mean weight-adjusted Erythropoietin resistance index (ERI) and Odds of the highest quartile of ERI in relation to participants’ demographic and clinical characteristics, N=85.
On multivariate binary logistic regression analysis, only MICS associated with the highest quartile of ERI. Participants with MICS had almost 6 times the odds of having the highest quartile of ERI (Adjusted OR 6.22 (1.59-24.4), p=0.009) (Table 3).
Discussion
In this study, we investigated the magnitude and correlates of ERI among 85 patients on maintenance HD at MNH in Dar es Salaam, Tanzania. In a multivariate logistic analysis, ERI significantly correlated with MIS. The correlation displayed a dose-response pattern with higher mean MIS observed among the upper quartiles of ERI. These findings are consistent with other studies [9,10,16]. Patients with inflammation, on long-term HD therapy, and those with MICS had significantly higher mean ERI.
A strong correlation between MIS, EPO dose, and responsiveness has been reported by Kaya et al. in a study done in Turkey [16]. This association was observed in both adult and geriatric haemodialysis patients however, the later group had relatively higher MIS and ERI. To the contrary, in our study age was inversely correlated with ERI. In the Turkish study, it was also noted that nutritional parameters (albumin and BMI) were linked with ERI. Malnutrition influences the responsiveness to EPO therapy [16]. Patients with protein-energy wasting have micronutrient deficiencies that can lead to anaemia and higher EPO requirements [8,9].
Hypo-responsiveness to EPO therapy results in higher degrees of anaemia observed among patients with MICS [8]. MICS is accompanied by elevated inflammatory markers (CRP and IL-6) that have been found to correlate with ERI, indicating the role of inflammation in the mechanism of EPO hypo-responsiveness [10]. High ERI may be viewed as an indirect measure of inflammation in HD patients that is in turn attributed to multiple factors like uremic toxins, dialysis procedure, biomembrane incompatibility, etc. [3,6,9,10].
Over three-fourths (78.8%) of the haemodialysis patients had iron deficiency, which is known to result from persistently high levels of hepcidin that accompanies worsening kidney function and inflammation [3,4]. Hepcidin downregulates ferroportin (transmembrane iron channel) present in enterocytes, macrophages, and hepatocytes. This hinders iron transport and releases from stores, thereby causing iron deficiency [3]. In our study, all patients were on conventional low flux HD that may not significantly clear hepcidin which is a middle-weight molecule [3].
Thus, MICS significantly contributes to the poor response to EPO therapy. If not treated, patients with MICS will continue requiring higher doses of EPO, which is detrimental to this subgroup of patients who already have varying degrees of cardiovascular impairment [12,17].
Contrary to other studies that have reported a link between ERI with transferrin saturation, and CRP, our study did not find these associations. This may be due to our small study sample as a limiting factor, as well as the influence of iron supplementation that is commonly practiced but not assessed in this study. In addition to that, we only assessed EPO dose over a short period of one month. We could not assess the hemoglobin change in relation to EPO dosing. This is because its evaluation requires a prospective follow-up of the weight-based EPO dose and hemoglobin level, which was impractical given the retrospective cross-sectional design of our study.
In this study, we excluded patients with chronic inflammatory conditions that are known to cause anaemia and subsequent poor response to EPO, however; we did not assess other factors like periodontal disease, nonfunctioning arteriovenous graft, and dialysate quality that are also known to induce inflammation [3].
As depicted above, inflammation has a significant influence on the response to EPO therapy as well as the nutritional status of HD patients. Therefore, anaemia that is resistant to standard EPO treatment warrants the search for underlying MICS. Several modalities in curbing inflammation in CKD patients have been reported. Statins have been shown to have anti-inflammatory effects and have also been reported to reduce EPO dose in HD patients [13]. Vitamin C supplementation has been also shown to minimize EPO requirements in HD patients [18]. Online hemodiafiltration has proven to reduce inflammation and improve EPO responsiveness through more effective clearance of middle-weight molecules [3]. Improving dialysis adequacy, the biocompatibility of membranes, and the use of ultrapure dialysate have also been shown to improve EPO responsiveness [19,20].
Conclusion
In hemodialysis patients, anaemia resistant to EPO therapy is linked to malnutrition inflammation complex syndrome. The later needs to be actively sought and appropriately treated in patients displaying suboptimal hemoglobin levels despite being on seemingly adequate EPO dosing.
Declaration
Availability of data and materials: The dataset of this study is available from the corresponding author on a reasonable request.
Acknowledgments: The authors would like to appreciate the renal unit at Muhimbili National Hospital for their support during the data collection.
Ethics approval and consent to participate: The Institutional Review Board of Muhimbili University of Health and Allied Sciences (MUHAS) Ethical committee approved this study. All participants provided written informed consent.
Authors’ contributions: PKB prepared the study design, collected and analyzed the data, and was the principal author. PJR and FFF participated in the study design and critically revised this manuscript. All authors have read and approved this manuscript.
References
- Zadrazil J, Horak P. Pathophysiology of anemia in chronic kidney diseases: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015; 159: 197-202.
- Ruggajo P, May S, Jonathan I, Mngumi J, Fredrick F. Anaemia in Patients on Haemodialysis Therapy in Dar es Salaam, Tanzania. Int J Nephrol Kidney Fail. 2019; 5: 1-6.
- Rosati A, Ravaglia F, Panichi V. Improving erythropoiesis stimulating agent Hyporesponsiveness in hemodialysis patients: the role of Hepcidin and Hemodiafiltration online. Blood Purification. 2018; 45: 139-146.
- McMurray JJ, Parfrey PS, Adamson JW, Aljama P, Berns JS, Bohlius J, et al. Kidney disease improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney International Supplements. 2012; 2: 279-335.
- López-Gómez JM, Portolés JM, Aljama P. Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney International. 2008; 74: S75-81.
- Costa E, Lima M, Alves JM, Rocha S, Rocha-Pereira P, Castro E, et al. Inflammation, T-cell phenotype, and inflammatory cytokines in chronic kidney disease patients under hemodialysis and its relationship to resistance to recombinant human erythropoietin therapy. Journal of clinical immunology. 2008; 28: 268-275.
- Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. American Journal of Kidney Diseases. 2020; 76: S1-S107.
- Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutritioninflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. American journal of kidney diseases. 2001; 38: 1251- 1263.
- Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. American journal of kidney diseases. 2003; 42: 761-773.
- Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, et al. Association of malnutrition–inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrology Dialysis Transplantation. 2013; 28: 1936- 1945.
- Santos EJ, Hortegal EV, Serra HO, Lages JS, Salgado-Filho N, Dos Santos AM. Epoetin alfa resistance in hemodialysis patients with chronic kidney disease: a longitudinal study. Brazilian Journal of Medical and Biological Research. 2018; 51: 1-7.
- Streja E, Park J, Chan TY, Lee J, Soohoo M, Rhee CM, et al. Erythropoietin dose and mortality in hemodialysis patients: marginal structural model to examine causality. International journal of nephrology. 2016; 2016: 1-8.
- Chiang CK, Yang SY, Peng YS, Hsu SP, Pai MF, Huang JW, et al. Atorvastatin increases erythropoietin-stimulating agent hyporesponsiveness in maintenance hemodialysis patients: role of anti-inflammation effects. American journal of nephrology. 2009; 29: 392-397.
- Bramania PK, Ruggajo P, Bramania R, Mahmoud M, Furia FF. Prevalence of malnutrition inflammation complex syndrome among patients on maintenance haemodialysis at Muhimbili National Hospital in Tanzania: a cross-sectional study. BMC nephrology. 2020; 21: 1-1.
- Kasvosve I, Delanghe J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clinical Chemistry and Laboratory Medicine (CCLM). 2002; 40: 1014-1018.
- Kaya T, Sipahi S, Karacaer C, Nalbant A, Varim C, Cinemre H, et al. Malnutrition-inflammation score, anthropometric indices, and erythropoietin requirement in geriatric hemodialysis patients. Turkish Journal of Geriatrics. 2015; 18: 3-9.
- Frank H, Heusser K, Höffken B, Huber P, Schmieder RE, Schobel HP. Effect of erythropoietin on cardiovascular prognosis parameters in hemodialysis patients. Kidney international. 2004; 66: 832-840.
- Attallah N, Osman-Malik Y, Frinak S, Besarab A. Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. American journal of kidney diseases. 2006; 47: 644-654.
- Movilli E, Cancarini GC, Zani R, Camerini C, Sandrini M, Maiorca R. Adequacy of dialysis reduces the doses of recombinant erythropoietin independently from the use of biocompatible membranes in haemodialysis patients. Nephrol Dial Transplant. 2001; 16: 111-114.
- Hsu PY, Lin CL, Yu CC, Chien CC, Hsiau TG, Sun TH, et al. Ultrapure dialysate improves iron utilization and erythropoietin response in chronic hemodialysis patients-a prospective cross-over study. J Nephrol. 2004; 17: 693-700.