Case Report
Austin J Neurol Disord Epilepsy. 2014;1(1): 3.
Giant Convexity Chondroma of the Dura Matter Presenting with Epilepsy
Sharafeddine H1, Elias E1, Jabbour M2, Najjar MW1*
1Departement of Surgery, American University of Beirut Medical Center, Lebanon
2Departement of Pathology, American University of Beirut Medical Center, Lebanon
*Corresponding author: Najjar MW, Department of Surgery, Division of Neurosurgery, American University of Beirut Medical Center, Riad El-Solh, 1107 2020, Beirut, Lebanon
Received: June 05, 2014; Accepted: July 11, 2014; Published: July 14, 2014
Abstract
Convexity chondromas are very rare benign extra-axial tumors that tend to present with protracted symptomatology, and as large non-enhancing dural based masses. We report one of the largest convexity chondromas that presented with 2 years of epilepsy, and review the pertinent literature especially as it relates to presentation, pathogenesis and management. We conclude that despite the rarity of the condition, convexity chondromas should be considered as part of the differential of large extra-axial lesions, especially when they are non-enhancing on MRI, and have a slowly progressive presentation. Surgical gross total removal is associated a good outcome.
Keywords: Cranial Chondroma; Extra-axial lesions; Giant extra axial tumors; Epilepsy
Background
Chondromas are rare intracranial tumors at an estimated incidence of 0.2-0.5% [1,2]. They usually arise from synchondrosis at the skull base, and are therefore seen mostly in sphenopetrosal, spheno-occipital and spheno-ethmoidal regions [3,4]. In extremely rare cases chondromas may arise from the convexity related to dura matter. To the best of our knowledge, 23 cases of chondromas originating from the convexity dura have been reported in literature. We present the case of a giant convexity chondroma with review of the literature for similar cases.
Clinical Presentation
This is a 25 years old man who started 2 years earlier to have focal seizures and was managed with anti-epileptic drugs, with partial success, until he presented to our emergency unit with partial seizures involving the right arm and leg, and was admitted for further investigation and possible surgery.
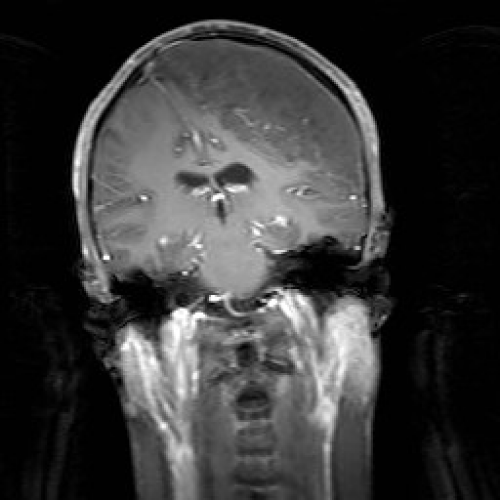
MRI revealed a large extra-axial mass along the left fronto-parietal convexity, showing heterogeneous signal intensity with areas of high T2 and low T1 signal intensity. It had a maximal thickness of 4.2 cm, and measured 11.9 x 12.3 cm in its maximal AP and transverse dimensions. Following contrast administration, there were flow voids within the lesion; however, there was no evidence of significant enhancement. The mass caused significant compression of the underlying frontal and parietal lobes, although there was no evidence of edema or abnormal signal. Along its medial aspect, the mass extended to the contralateral convexity, displacing the falx and superior sagittal sinus to the right (Figure 1). MRA and MRV were done, and there was lack of opacification of the superior sagittal sinus over a length of approximately 9.8 cm, probably related to invasion or compression by the mass.
Figure 1: Coronal T1W MRI section with Gadolinium showing a large extraaxial mass along the left fronto-parietal convexity, showing heterogeneous signal intensity with flow voids within the lesion, but no significant enhancement. The mass causes significant compression of the underlying frontal and parietal lobes, although there is no evidence of edema or abnormal signal. Along its medial aspect, the mass extends to the contralateral convexity, displacing the falx and superior sagittal sinus to the right.
After performing multiple burr holes, we carried out a large craniotomy flap crossing the midline. The superior sagittal sinus was displaced by few centimeters to the right side. The dura was opened and a hard cartilaginous like mass was discovered and removed slowly, lifting it from the underlying cortex, which was severely compressed. There was no gross evidence of invasion. A large periosteal flap was used to graft the dural defect, and then closure was performed as usual. The patient recovered well and remained stable clinically at last follows up.
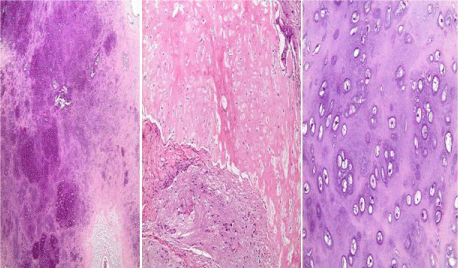
Gross examination revealed a 13 x 12 x 4 cm tan-white mass with a smooth convex contour in close proximity to the skull and an irregular concave border adjacent to the brain (Figure 2). The cut-surface was irregular, firm, and homogenous with occasional cystic spaces. Microscopically the mass was composed entirely of cartilaginous lobules formed of mildly pleomorphic chondrocytes with rare binucleated forms. Mitotic figures were not identified. At the brain-tumor interface, the cartilage formed a discrete boundary with foci of vimentin-positive meningothelial cells (Figure 3).
Figure 2: Gross image of the giant chondroma showing a cartilaginous cutsurface with intermixed cystic spaces. The irregular contour represents the area compressing the brain.
Figure 3: H&E stained sections of the giant chondroma characterized by cartilaginous lobules and myxoid cystic areas (left, mag. 40x) which are sharply separated from the brain matter with interspersed meningothelial cells (middle, mag. 100x). The high-power image highlights the mild pleomorphism with occasional binucleation (right, mag. 200x).
The cartilaginous lesion morphology favored the diagnosis of a chondroma; however a low-grade chondrosarcoma could not be entirely excluded due to the large size of the lesion and the presence of mild cytological atypia. A 2-month follow-up period associated with a recurrence-free interval would probably support the diagnosis of a giant chondroma.
Discussion
Intracranial chondromas are extremely rare entities. These lesions were described for the first time by Hirschfield in 1851 [5]. Chondroma arising from convexity or the falx is even a rarer entity, comprising 20% of all intracranial chondromas [4]. Upon review of the literature, 25 cases of intracranial dural convexity chondromas have been reported, and we describe the largest (11.9X12.3cm) intracranial chondroma yet.
Intracranial chondromas may be solitary, or they may occur as multiple lesions with an increased risk of malignant change, as in the case of Ollier's disease or Maffucci's syndrome [6]. In this report, a young man was investigated for right sided headache, visual disturbances and Jacksonian seizures. He had deformities in all four limbs on examination along with temporal hemianopsia, and bilateral exophthalmos more dominant on the left side. He also had diplopia and bilateral horizontal nystagmus. A left carotid angiogram showed an aneurysm arising from the paraophthalmic region of the left internal carotid artery. TI-weighted MRI brain showed a mass lesion in the sphenoid bone and clivus. Partial excision of the mass was attempted and an enchondroma consisting of cartilaginous tissue of the hyaline variety without any sarcomatous change found on pathology. No follow up was mentioned by the authors.
More recently, a case of a dural convexity chondroma was reported in a subject with Noonan's syndrome [7]: the patient was a young male, with history of single generalized seizure, already confirmed to have Noonan's syndrome. MRI of the brain revealed a dural convexity intracranial mass, well circumscribed, and positioned in the left fronto parietal region. He underwent craniotomy for resection. His follow up was reported for 33 months with no evidence of disease recurrence on MRI. Pathology report revealed a mature chondroma.
Various theories have been proposed for the origin of intracranial chondromas, but the pathogenetic mechanism of the disease remains controversial. Four speculative hypotheses on the histogenesis of dural chondromas have been proposed:
- Development from heterotopical chondrocytes [8].
- Metaplasia of meningeal fibroblasts [1,9].
- Metaplasia of perivascular mesenchymal tissue [10].
- Origin from meningeal connective tissue, as result of cartilaginous activation of fibroblasts by trauma or inflammation [11].
These tumors usually differ from meningeomas. They occur at ages between 20 and 60 with no gender predominance [12]. The patients usually present with long time history of symptoms and signs because they are slow growing tumors. At time of presentation, most of the tumors are usually large. The clinical presentation of convexity or falcine chondromas is nonspecific, and the symptomatology of these chondromas is associated with their anatomic location. Manifestations of the mass are related to dysfunctions that are secondary to either local parenchyma compression, epileptic seizures or increased intracranial pressure [13,14].
Neuroimaging for intracranial chondromas is almost typical. On CT scan, the tumor's density is variable, reflecting differences in the degree of calcification [5,8]. The tumor may either be hyperdense or hypodense, and bone imaging may show either an erosion or hyperostosis of the inner table of the skull [13]. Peritumoral edema is usually rare [15]. MRI may reveal a tumor appearing hypo- or isointense on T1-weighted images and a mixture of hypo- and hyperintense appearance on T2-weighted sequences. They do not display any dural tail signs and are usually non-enhancing [16].
On cerebral angiograms, an avascular extracerebral space-occupying lesion is usually revealed with displacement or compression of arteries in the vicinity of the tumor. Surgical resection is the best treatment for intracranial chondroma. These tumors are well defined and don't invade the surrounding brain tissue. In case of convexity tumors, it is recommended to remove overlying dura as well [12].
Recurrence is unlikely after total resection, therefore a favorable prognosis is expected [4,13]. Chondromas don't respond to radiotherapy; on the contrary radiation may induce malignant transformation [8]. Therefore there is no role for radiotherapy in cases of incomplete resection, and the best solution is to target a complete resection during surgery, where long term remission may be then expected [17].
Conclusion
Intracranial chondromas are rare benign tumors that arise from skull base, but may be convexity lesions as well. Chondroma may be considered as part of the differential diagnosis when treating extra-axial tumors of the convexity, especially when the lesion is large, non-enhancing, and with slowly progressive symptomatology such as epilepsy, as we have briefed in this report. Surgical gross removal is associated with a good outcome.
References
- Berkmen YM, Blatt ES. Cranial and intracranial cartilaginous tumours. Clin Radiol. 1968; 19: 327-333.
- Kretzschmar HA, Eggert HR, Beck U, Fürmaier R. Intracranial chondroma. Case report. Surg Neurol. 1989; 32: 121-125.
- Erdogan S, Zorludemir S, Erman T, Akgul E, Ergin M, Ildan F, et al. Chondromas of the falx cerebri and dural convexity: report of two cases and review of the literature. J Neurooncol. 2006; 80: 21-25.
- Fountas KN, Stamatiou S, Barbanis S, Kourtopoulos H. Intracranial falx chondroma: literature review and a case report. Clin Neurol Neurosurg. 2008; 110: 8-13.
- Dutton J. Intracranial solitary chondroma. Case report. J Neurosurg. 1978; 49: 460-463.
- Ghogawala Z, Moore M, Strand R, Kupsky WJ, Scott RM. Clival chondroma in a child with Ollier's disease. Case report. Pediatr Neurosurg. 1991; 17: 53-56.
- Delgado-Lopez PD, Martin-Velasco V, Galacho- Harriero AM, Castilla-Diez JM, Rodriguez-Salazar A, Echevarria-Iturbe C. Large chondroma of the dural convexity in a patient with Noonan's syndrome. Case report and review of the literature. Neurocirugia (Astur). 2007; 18: 241-246.
- Russell DS. Meningeal Tumours: a Review. J Clin Pathol. 1950; 3: 191-211.
- Acampora S, Troisi F, Fusco G, Del Gaizo S. Voluminous intracranial chondroma. Surg Neurol. 1982; 18: 254-257.
- Ahyai A, Spoerri O. Intracerebral chondroma. Surg Neurol. 1979; 11: 431-433.
- Chorobski J, Jarzymski J, Ferens E. Intracranial solitary chondroma. Surg Gynecol Obstet. 1939; 68: 677-686.
- Nakayama M, Nagayama T, Hirano H, Oyoshi T, Kuratsu J. Giant chondroma arising from the dura mater of the convexity. Case report and review of the literature. J Neurosurg. 2001; 94: 331-334.
- Laghmari M, Metellus P, Fuentes S, Adetchessi T, Dufour H, Bouvier C, et al. [Cranial vault chondroma: a case report and literature review]. Neurochirurgie. 2007; 53: 491-494.
- Patel A, Munthali L, Bodi I. Giant cystic intracranial chondroma of the falx with review of literature. Neuropathology. 2009; 29: 315-317.
- Brownlee RD, Sevick RJ, Rewcastle NB, Tranmer BI. Intracranial chondroma. AJNR Am J Neuroradiol. 1997; 18: 889-893.
- Khosrovi H, Sadrolhefazi A, el-Kadi H, Bloomfield SM, Schochet SS. Intradural convexity chondroma: a case report and review of diagnostic features. W V Med J. 2000; 96: 612-616.
- Hardy RW Jr, Benjamin SP, Gardner WJ. Prolonged survival following excision of dural chondroma. J Neurosurg. 1978; 48: 125-127.