Case Report
Austin J Neurol Disord Epilepsy. 2014;1(1): 3.
Multiple Meningiomas of Different Histologic Subtype: Case Series and Literature Review
Elizabeth Klein*, Paris Humphries, William Kupsky, Murali Guthikonda and Nicholas Szerlip
Department of Neurological Surgery, Wayne State University Detroit, USA
*Corresponding author: Elizabeth Klein, Department of Neurosurgery, Wayne State University, 4160 John R, Suite 6E, Detroit MI 48201, Michigan, USA
Received: June 27, 2014; Accepted: July 28, 2014; Published: July 30, 2014
Abstract
Meningioma is the most common primary CNS tumor. Multiple meningiomas (MM) are rare outside of neurofibromatosis, and MM of different histologic subtypes is even less common. Here we describe two cases of MM with different histology and review the literature on MM. Two hundred twenty five publications on MM were found through a Medline search. Only six describe meningiomas of different histologies. Our series is the first that we know of to describe MM that includes the secretory subtype.
Keywords: Multiple meningioma; Meningioma subtype; Histopathology
Introduction
Meningiomas are the most common primary, non-glial tumors of the CNS accounting for 30% of all CNS tumors. The term "multiple meningiomas" (MM) was first defined by Cushing and Eisenhardt in 1938 as "at least two spatially separated meningiomas in a patient without signs of neurofibromatosis". MM are found in approximately one to ten percent of all meningioma patients. Neurofibromatosis type 2 (NF2) is the most common genetic syndrome implicated in MM, however other genetic factors have been implicated in MM as well [1]. The sparsity of sporadic MM leads to a paucity of data about their natural history and clinical significance. Prior studies have shown no statistical correlation of tumor growth rate with the total number of meningiomas [2]. Only six prior case studies have described patients with MM of different histology and none of these has described MM of secretory subtype. Here, we present two cases of multiple intracranial meningiomas of different histologic subtype and review the relevant literature to date.
Case 1
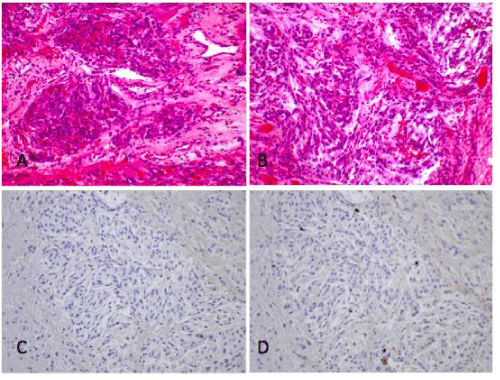
The first patient is a 41-year-old female who presented with complaints of progressive vision loss and diplopia over the previous year. Exam revealed right proptosis, but no neurological deficits. She underwent MRI of the brain, which demonstrated several well-defined, homogenously enhancing extra axial masses located on the dorsal surface of the tentorium, left frontal convexity, and right middle cranial fossa, extending into the optic canal and involving the cavernous sinus (Figure 1). She was taken to the operating room for right frontotemporal craniotomy with excision of the right middle fossa lesion to alleviate her symptoms and prevent any further vision loss. Histopathologic sections showed nests of tumor cells in a loose vascular stroma with cytoplasmic globular vacuoles, with positive immunoreactivity to carcino embryonic antigen and progesterone receptor. These findings are consistent with meningioma, secretory type, WHO grade I (Figure 2).
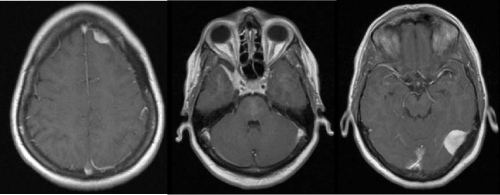
Figure 1: Axial postcontrast MRI with three well-defined enhancing lesions consistent with meningiomas, located on the left tentorium, in the left frontal region, and the medial aspect of the right middle cranial fossa along the sphenoid wing and cavernous sinus.
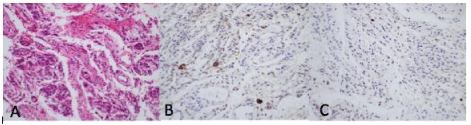
Figure 2: Histopathologic sections of secretory meningioma.A, H&E stain with nests of tumor cells in a loose vascular stroma with cytoplasmic globular vacuoles. B, CEA stain shows positive immunoreactivity. C, Ki-67 labeling index is low.
Sixteen months later, the patient underwent a second craniotomy for resection of the left tentorial mass. The tumor extended from the transverse-sphenoid junction along the transverse sinus and invaded the cerebellum. Histopathologic examination revealed again meningioma, secretory type, WHO Grade I.
Approximately two years later, the patient underwent a third operation for excision of the left frontal mass. The patient had noted swelling over the left eye and visual complaints. The left frontal lesion had grown on repeat imaging and was surgically resected. In this case, the histopathologic specimen demonstrated a predominantly meningothelial pattern, with focal microcystic areas rather than the secretory pattern seen in the previous tumor specimens. The tumor cells were immunoreactive for endothelial membrane antigen (EMA) and PR, but were not reactive for CEA (Figure 3).
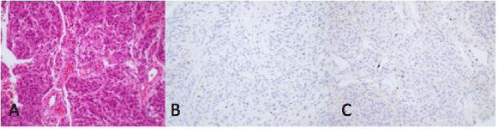
Figure 3: Histopathologic sections of meningothelial meningioma.A, H&E stain demonstrating cells arranged in a meningothelial pattern with focal microcystic areas. B, CEA stain is negative. C, Ki-67 labeling index is low.
Postoperatively the patient remained free of neurologic deficits. She did have issues with wound healing after her multiple craniotomies, requiring subsequent operations for grafting and placement of free flaps.
Case 2
The second patient is a 52-year-old female who initially presented to the neurology clinic with headache and blurred vision in the right eye. On exam, she had right proptosis and was otherwise neurologically intact. MRI brain was ordered by her neurologist, which demonstrated three extra axial enhancing lesions. The first lesion occupied the right cavernous sinus and extended into Meckel's cave and the superior orbital fissure. The other two lesions were found over the right and left frontal convexities (Figure 4). This patient was admitted and underwent resection of the cavernous sinus lesion with decompression of the orbital portion and optic nerve. The right convexity tumor was also removed at this time. Resection of the left convexity lesion was planned at a later date.
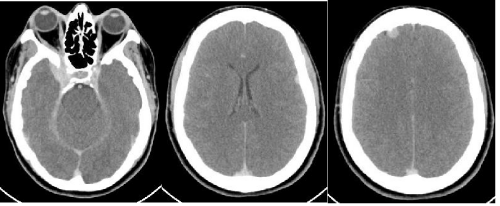
Figure 4: Axial CT demonstrating extraaxial masses consistent with meningioma in the right cavernous sinus, left posterior frontal region, and right anterior frontal region.
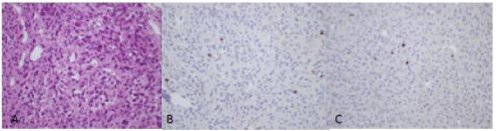
Two separate pathologic specimens were sent. The section from the cavernous sinus lesion demonstrated pieces of fibrous tissue containing nests with occasional whorl formations and scattered intranuclear pseudoinclusions, consistent with meningothelial meningioma (Figure 5). The specimen from the right convexity tumor demonstrated scattered CEA-immunoreactive globules in a loose architecture, consistent with secretory meningioma with meningothelial features (Figure 6). The patient did well postoperatively and recovered without any neurologic deficits.
Figure 5: Histopathology of meningothelial meningioma. A, B, H&E stain demonstrating meningothelial pattern. C, CEA shows no immunoreactivity. D, Ki-67 labeling index is low.
Figure 6: ASecretory meningioma with meningothelial features.A, H&E stain demonstrating a loose pattern with secretory globules. B, CEA stain demonstrates positive reactivity in globules. C, Ki-67 labeling index is low.
Literature Review
A Medline search for "multiple meningiomas" returned 225 results. Publications were limited to those published in English after 1980. Articles were excluded if they did not focus centrally on MM, focused on spinal MM, pediatric MM, or MM in the setting of prior irradiation. Articles about MM in the setting of NF2 were also excluded. After filtering for those exclusion criteria, six publications discussed MM of different histological subtypes in a single patient, not in association with NF2. None of these included secretory meningioma (Table 1).
Author
Publication date
Number of cases included
Histology
Klein
2014
2
Secretory
Meningothelial
Sriram
2013
1
Chordoid
Meningothelial
Mocker
2011
1
WHOI/WHOII
Ge
2010
1
Fibrous
Psammomatous
Huang
2005
7
Unknown
Tomita
2003
1
Fibrous
Anaplastic
Koh
2001
1
Atypical
Psamommatous
Table 1: Summary of case reports of MM of different pathologic subtypes.
In 2001, Koh et al published a case report describing a patient with MM of benign and malignant pathology. One tumor was a paramedian-located atypical meningioma and the other a left convexity psammomatous meningioma [3]. Two years later, Tomita et al. reported a single patient having both a fibrous meningioma and anaplastic meningioma in the same hemisphere [4]. Huang et al examined retrospective data in 2005and reported a total of 19 patients who had more than one subtype of meningioma resected. This study however, did not discuss specific histologies for each of the 7 patients, and looked at the relative distribution of subtypes among the total number of meningiomas resected [5]. In 2010, Ge et al published a case study of a male patient who had MM located at the sphenoid ridge. Histology confirmed one fibrous meningioma and one psammomatous meningioma [6]. In 2011, Mocker et al. carried out a detailed genetic analysis in a patient with MM [7]. The patient had both WHO grade I and II meningiomas concurrently. Both grades shared terminal deletions on chromosome 1p and/or monosomy of chromosome 22. The higher grade meningioma however, also had a paracentric inversion within 1p36. The two grades shared a majority of genetic atypia, but not all. More recently in 2013, Sriram reported a patient with a right sphenoid wing chordoid meningioma and left frontal meningotheliomatous meningioma [8]. There were no prior case reports documenting chordoid meningioma in MM patients. As the author pointed out, chordoid meningiomas have higher propensity to recur and MM cases with these tumors must be managed more aggressively. To date, there has not been a case report describing two different patients with the same two subtypes of MM; secretory and meningothelial.
Discussion
Meningiomas are slow growing; extra axial tumors that arise from arachnoid cap cells are well known. The most common locations are cerebral convexity (35%), parasagittal (20%), sphenoid ridge (20%), infratentorial, (13%), intraventricular (5%), and tuberculum sellae (3%). These tumors have a slight female preponderance (2:1) and incidence increases with age [1].
Most meningiomas are sporadic; however there is an increased incidence in patients who have undergone radiation to the scalp or teeth. Patients with neurofibromatosis type 2 have a 50% chance of developing meningioma, and other genetic factors leading to predisposition to meningioma have also been identified (ref). Many meningiomas are asymptomatic throughout a patient's life, and about 1-2% of autopsies reveal incidental meningioma.
The 2000 WHO classification divides meningiomas into three grades based on their pathological features, risk of recurrence, and aggressiveness of growth. Grade I includes meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secrectory, lymphoplasmacyte-rich, and metaplastic subtypes. Grade II includes clear cell, chordoid, and atypical types. Grade III are the most aggressive and include rhabdoid, papillary and anaplastic types. Meningothelial meningioma is widely regarded as the most common histolopathological subtype.
The incidence of MM has been reported as anywhere from one to ten percent. They are thought to develop from a single precursor via the subarachnoid space. In cases of multiple meningiomas, the most commonly reported subtypes have been meningothelial, transitional, psammomatous, fibroblastic [9,10]. Progesterone receptor expressivity is higher in MM than in solitary meningiomas (Honguang). Sixty to 90% of MM is found in women [1]. MM generally carry the same prognosis as solitary meningiomas and treatment options are similar, however it has been noted that recurrence rate may be higher in cases of MM [11].
In our case series, both patients with MM had meningothelial and secretory subtypes. Although meningothelial meningioma is very common in MM, the secretory subtype is rarer. The variability in histopathology suggests that these tumors may have come from different molecular precursors, which negates the common thought that MM arise from spread through the subarachnoid space. It is uncertain whether this has any prognostic significance for these patients; however it does call into question the underlying mechanism of disease development of MM. Further genetic profiling may be of use in these patients to identify any common underlying factors contributing to this condition.
Conclusion
Although meningiomas are very common, MM is still quite rare outside of NF2, and those with different histopathologic characteristics are even more unique. Although the prognosis and treatment is generally similar for MM as compared to solitary meningiomas, this information causes us to reconsider the underlying mechanism of MM development.
References
- Youmans Neurological Surgery. Sixth edition. Elseiver 2011.
- Wong RH, Wong AK, Vick N, Farhat HI. Natural history of multiple meningiomas. See comment in PubMed Commons below Surg Neurol Int. 2013; 4: 71.
- Koh YC, Yoo H, Whang GC, Kwon OK, Park HI. Multiple meningiomas of different pathological features: case report. See comment in PubMed Commons below J Clin Neurosci. 2001; 8 Suppl 1: 40-43.
- Tomita T, Kurimoto M, Yamatani K, Nagai S, Kuwayama N, Hirashima Y, et al. Multiple meningiomas consisting of fibrous meningioma and anaplastic meningioma. See comment in PubMed Commons below J Clin Neurosci. 2003; 10: 622-624.
- Huang H, Buhl R, Hugo HH, Mehdorn HM. Clinical and histological features of multiple meningiomas compared with solitary meningiomas. See comment in PubMed Commons below Neurol Res. 2005; 27: 324-332.
- Ge PF, Fu SL, Liu DH, Zhong YP, Luo YN. Two subtypes of meningiomas with different imaging co-existed at sphenoid ridge. See comment in PubMed Commons below Br J Neurosurg. 2010; 24: 720-721.
- Mocker K, Holland H, Ahnert P, Schober R, Bauer M, Kirsten H, et al. Multiple meningioma with different grades of malignancy: case report with genetic analysis applying single-nucleotide polymorphism array and classical cytogenetics. Pathol Res Pract. 2011; 207: 67-72.
- Sriram PR. Chordoid meningioma, part of a multiple intracranial meningioma: a case report & review. See comment in PubMed Commons below Malays J Med Sci. 2013; 20: 91-94.
- Gelabert-González M, Leira-Mui&nTilde;o R, Fernández-Villa JM, Iglesias-Pais M. [Multiple intracranial meningiomas]. See comment in PubMed Commons below Rev Neurol. 2003; 37: 717-722.
- Koech F, Orege J, Ndiangui F, Macharia B, Mbaruku N. Multiple intracranial meningiomas: a review of the literature and a case report. See comment in PubMed Commons below Case Rep Surg. 2013; 2013: 131962.
- Boylan SE, McCunniff AJ. Recurrent meningioma. See comment in PubMed Commons below Cancer. 1988; 61: 1447-1452.