
Review Article
Austin J Neurol Disord Epilepsy. 2015; 2(1): 1010.
Receptors of Chondroitin Sulfate Proteoglycans and CNS Repair
Yosuke Ohtake and Shuxin Li*
Department of Anatomy and Cell Biology, Temple University School of Medicine, USA
*Corresponding author: Shuxin Li, M.D. PhD., Department of Anatomy and Cell Biology, Shriners Hospital’s Pediatric Research Center, Temple University School of Medicine, 3500 N. Broad Street, Philadelphia, PA, 19140, USA
Received: March 15, 2015; Accepted: June 02, 2015; Published: June 08, 2015
Abstract
Axon disconnections in the CNS usually cause persistent dysfunction with a very limited recovery and the medical treatments to enhance recovery from neurological deficits due to signal conduction failure are largely restricted. Among numerous factors that contribute to regenerative failure of CNS axons, the extracellular matrix molecules Chondroitin Sulfate Proteoglycans (CSPGs) generated by scar tissues are critical for blocking axon elongation following CNS injuries. Overcoming inhibition by CSPG axon growth inhibitors is very important for promoting successful axon regeneration and functional recovery after CNS injuries. Recent progress in understanding of molecular mechanisms underlying CSPG suppression of neuronal growth may facilitate development of new treatments to surmount scar- mediated inhibition. Particularly, a number of studies demonstrate that CSPG inhibitors convey their suppression of axon growth by interacting with several neuronal transmembrane receptors. Two members of the Leukocyte Common Antigen Related (LAR) phosphatase subfamily, protein tyrosine phosphatase σ and LAR, bind CSPGs with high affinity and mediate CSPG inhibition as functional receptors. CSPGs appear also to bind two receptors for myelin-associated growth inhibitors, Nogo receptors 1 and 3. Transgenic or pharmacological blockade of these receptors significantly surmounts CSPG function and promotes CNS axon regeneration. Identification of the CSPG receptors is likely to facilitate developing novel and selective therapies to promote axon sprouting/regeneration and functional recovery after CNS injuries.
Keywords: Axon regeneration; CNS injury; Reactive glial scar; CSPG receptor; LAR; PTPσ; Nogo receptor
Introduction
Following CNS injuries, astrogliosis is a defense response to minimize and repair primary damage, including isolating intact tissue from secondary lesions, maintaining a favorable environment for surviving neurons, preserving the blood brain barrier (BBB), generating permissive substrates for neurite extension and other protective effects (Karimi-Abdolrezaee and Billakanti, 2012; Sofroniew, 2009). Ablation of reactive astrocytes or interfering with their activation could exacerbate tissue damage after Spinal Cord Injury (SCI) by increasing tissue degeneration and failing to reconstruct BBB (Faulkner et al., 2004; Sofroniew, 2009). However, the reactive glial scars ultimately generate detrimental effects due to forming both physical and chemical barriers to axon regeneration, including producing high levels of inhibitory molecules to suppress neuronal regeneration. Proliferation and migration of a large number of reactive astrocytes into and around the lesion areas and formation of glial scar tissues constitute physical barrier of axon regeneration. Upregulation of suppressing substances strongly hinders axon regeneration and neural repair and the inhibitory properties of reactive astrocytes develop with time after CNS injuries.
The integrations between growth-promoting Extracellular Matrix (ECM) molecules (such as laminin, fibronectin and integrins) and growth-suppressing molecules are essential for determining elongation or termination of lesioned CNS axons. Glial scar is a major detriment to regeneration of severed axons by upregulating a great number of molecules around the lesion and preventing regrowth of injured axons at the lesion area, including Chondroitin Sulfate Proteoglycans (CSPGs), tenascin, semaphorin 3A, Keratan Sulfate Proteoglycans (KSPGs), myelin-associated inhibitors and Ephrins/ Eph receptors. Among them, CSPGs are an extremely important class of growth inhibitors highly upregulated by scar tissues. CSPGs are a family of molecules characterized by a core protein to which the large and highly sulfated Glycosaminoglycan (GAG) chains are attached. The major CSPGs found in the CNS include lecticans (neurocan, versican, aggrecan and brevican), phosphacan (6B4 proteoglycan) and NG2. CSPGs are concentrated into perineuronal nets (PNNs), which are mainly composed of hyaluronan, CSPGs, tenascin R and link proteins. Evidence for the inhibitory nature of CSPGs on axon regeneration came largely from studies on digestion of GAG side chains of CSPGs with the bacterial enzyme Chondroitinase ABC (ChABC). Although CSPG core proteins are inhibitory by themselves (Oohira et al., 1991; Tan et al., 2006), removal of GAG side chains with ChABC makes the ECM environment much more permissive to axon elongation (Crespo et al., 2007) and promotes axon sprouting or regeneration after CNS injury. CSPGs have been found to be inhibitory for over 25 years (McKeon et al., 1991; Snow et al., 1990; Snow et al., 1991), but molecular mechanisms for them to suppress neuronal growth are not well known. One of the major advances in the scar-mediated inhibition on neuronal growth is identification of several functional receptors for CSPGs, especially two members in the Leukocyte Common Antigen Related (LAR) subfamily of Receptor Protein Tyrosine Phosphatases (RPTPs) (Fisher et al., 2011; Shen et al., 2009). In this review, we will focus on recent progress in the receptors of scar-sourced axonal growth inhibitors and the therapeutic potential for blocking CSPGs and their receptors.
Previous view of axon growth inhibition by CSPGs
A few mechanisms had previously been thought to attribute to CSPG inhibition of neuronal growth. CSPGs are the large sizes of molecules and are concentrated into PNNs with several other ECM molecules. Interactions between the PNN molecules produce a stable pericellular complex around synapses and play a vital role in controlling reduced plasticity of developed neurons (Kwok et al., 2011). CSPGs had been proposed to hinder the growth-promoting adhesion ECM molecules sterically, which are important regulators of neuronal adhesion and growth. As the transmembrane receptors for ECM molecules such as laminin, integrins function as cell surface adhesion molecules and link them to actin cytoskeleton. The highly charged GAG moieties of CSPGs can interact with these ECM molecules and suppress neurite growth by attenuating integrin activation (Afshari et al., 2010; Tan et al., 2011). Over- expression of integrins could overcome CSPG inhibition of axon growth (Condic et al., 1999). Thus, CSPGs reduce activity of integrin signaling pathway and activation of integrin signaling overcomes inhibition by CSPGs.
The lectican CSPG aggrecan suppresses laminin-mediated growth of cultured rat sensory neurons without altering surface integrin levels by reducing levels of phosphorylated focal adhesion kinase and Src (Tan et al., 2011). Activation of integrin signaling by applying manganese or an activating antibody surmounts aggrecan inhibition on elongation of cultured neurons. Over-expression of kindlin-1, a phosphoprotein involved in attachment of actin cytoskeleton to plasma membrane and integrin-mediated function, activates integrin signaling and enhances growth of sensory neurons cultured on aggrecan and regeneration of injured sensory axons across the dorsal root entry zone and into the spinal cord (Tan et al., 2012). Over-expression of growth-associated protein-43 and/or β1 integrin could partly stimulate regeneration of serotonergic axons on high levels of CSPG and blockade of β1 integrin reduced serotonergic and cortical outgrowth on laminin (Hawthorne et al., 2011). Notably, the functional link between laminin/integrins and CSPGs is not specific because integrin activation also inverted neuronal growth suppression by myelin associated inhibitors (Tan et al., 2011).
CSPGs could contribute to inhibition of neuronal growth by certain chemo-repulsive proteins. The thrombospondin repeats of Sema5A, an axon guidance cue, bind the GAG chains of both CSPGs and Heparan Sulfate Proteoglycans (HSPGs) and these interactions could convert Sema5A from an attractive to an inhibitory guidance cue (Kantor et al., 2004). Sema3A, another repulsive guidance molecule, could interact with chondroitin sulfate-4,6 enriched in the PNNs and this interaction appear to regulate the repulsive function of Sema3A (De Wit et al., 2005; Deepa et al., 2006; Kwok et al., 2011). Moreover, CSPGs may modulate neuronal growth by binding extracellular calcium or its channels and affecting calcium availability and entry into neurons (Hrabetova et al., 2009). Therefore, neuronal growth is partially mediated by the ratio between growth-promoting (such as laminin) and growth-inhibiting (such as CSPGs) molecules present in the environment (Snow et al., 2002).
Important function of CSPG receptors on neuronal growth
The molecular mechanisms for CSPG suppression of neuronal growth are not well understood although CSPGs have been known to hinder neuronal regeneration and plasticity for over two decades (McKeon et al., 1991; Snow et al., 1990; Snow et al., 1991). So far, a number of mechanisms for CSPG functions have been supported, including binding to functional receptors on the neuronal membrane, establishing a non-permissive PNNs that causes steric hindrance of growth-promoting adhesion molecules (such as laminin and integrins) and facilitating function of some chemorepulsive molecules. Because preventing GAG sulfation of CSPGs removes much of their inhibitory activity on axon growth in vitro (Gilbert et al., 2005; Sherman and Back, 2008; Wang et al., 2008), the GAG sulfation patterns are important for CSPG function. CSPGs might non-specifically impede binding of some ECM molecules to their cell surface receptors through steric interactions, but recent reports demonstrate that two transmembrane proteins of the LAR phosphatase subfamily, Protein Tyrosine Phosphatase σ (PTPσ) and LAR, function as the receptors by binding CSPGs with high affinity and mediating CSPG inhibitory effects (Figure 1). (Fisher et al., 2011; Shen et al., 2009; Xu et al., 2015). Furthermore, CSPGs may act by binding to Nogo Receptor 1 (NgR1) and NgR3, two receptors known for myelin-associated inhibitors (Dickendesher et al., 2012). Thus, CSPGs block axon regeneration likely by multiple molecular mechanisms, making them especially potent and difficult therapeutic targets.
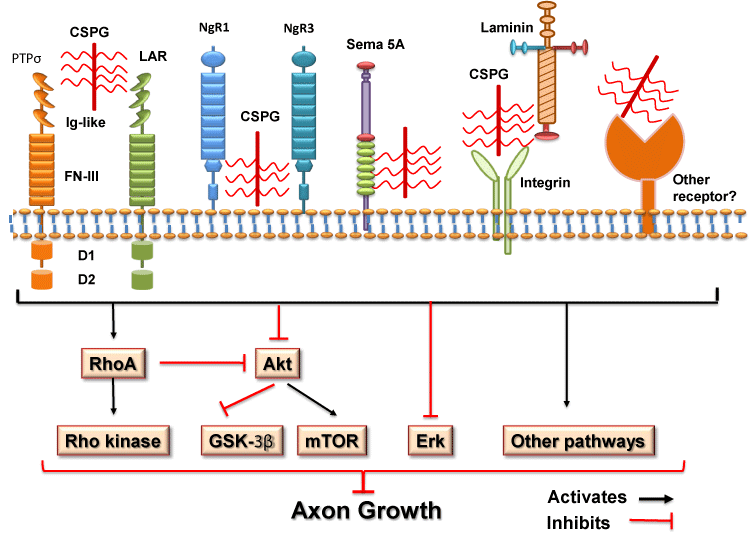
Figure 1: Schematic of the molecular mechanisms for CSPG inhibition on
neuronal growth and the downstream signaling pathways. CSPGs inhibit
neuronal growth by binding and activating several receptor proteins, including
PTPs, LAR, NgR1 and NgR3. CSPGs contribute to inhibitory properties of the
guidance molecule Sema 5A by converting it from an attractive to an inhibitory
cue. CSPGs may suppress axon growth by blocking function of growthpromoting
molecules, such as laminin and its receptor integrins. Also, CSPGs
might suppress neuronal growth through other unidentified transmembrane
receptors. Intracellularly, interactions between CSPGs and receptors/other
proteins activate RhoA-Rho kinase signaling and inactivate Akt and Erk
pathways. Activation and/or inactivation of these signaling pathways mediate
suppression of CSPGs through other downstream signals, including GSK-3β
and mTor. RhoA might also regulate PTEN activity and suppress neuronal
growth by inactivating mTOR signaling. Ig-like: immunoglobulin-like domains;
FN-III: fibronectin Type III domains; D1: D1 domain; D2: D2 domain.
LAR subfamily of phosphatases as CSPGs receptors
Most inhibitory molecules for neuronal growth suppress elongation mainly by binding and activating their specific functional receptors on membrane. A major progress in recent years is the identification of two receptor PTPs as functional receptors for CSPGs (Fisher et al., 2011; Shen et al., 2009; Xu et al., 2015). RPTPs are a major family of evolutionarily-conserved synaptic adhesion molecules and contain the common receptor-like structures, including the large extracellular adhesion-like domains (3 Ig-like domains and variable fibronectin type III repeats), a transmembrane region and two tandem PTP domains (catalytically active D1 and inactive D2). RPTPs could regulate the levels of intracellular tyrosine phosphorylation and presynaptic development by binding distinct synaptic membrane proteins and shaping various synaptic adhesion pathways. RPTPs exhibit a distinct spatial pattern of expression during development and may modulate axon growth and guidance in CNS (Bixby, 2000; Stoker, 2001). Some RPTPs exhibit certain spatiotemporal distribution in the CNS and are implicated in neuronal proliferation, differentiation, axon innervation and arborization (Reinhard et al., 2009).
The LAR subfamily includes 3 vertebrate homologs: LAR, PTPσ, and PTPd, which share 66% amino acid identity in the full-length proteins and 84% identity in the catalytic domains. Transgenic mice lacking these proteins exhibit some morphological and functional deficits. LAR -/- and +/- mice are viable and grossly normal in appearance, but the number of progeny in LAR deletion mice is lower than in wild type mice (17 vs. 25%) and LAR -/- mice have smaller basal forebrain cholinergic neurons and less cholinergic innervation of their target neurons in the dentate gyrus than controls (Yeo et al., 1997). Mice with deleted LAR phosphatase domains have spatial learning deficiency and hyperactivity (Kolkman et al., 2004). PTPσ -/- mice have severe growth retardation, high neonatal mortality and numerous neurological defects, including motor dysfunction, defective proprioception, hippocampal dysgenesis, abnormal pituitary development, and thinning of the corpus callosum and cerebral cortex (Meathrel et al., 2002; Uetani et al., 2006). PTPd knockout mice also exhibit obvious motor dysfunction and diminished visuospatial processing with low survival rates (Uetani et al., 2000; Uetani et al., 2006) although this RPTP has not been linked to CSPG function.
Before discovery of the CSPG receptors, several previous reports indicated strong structural and functional interactions between RPTPs and the GAG chains of certain proteoglycans. The first Iglike domain of PTPσ homologs bound the heparan sulfate GAG chains of agrin and collagen XVIII and stimulated growth of retinal ganglion neuron (Aricescu et al., 2002; Ledig et al., 1999). Drosophila LAR bound syndecan and Dallylike, two HSPGs, with high affinity and regulated synaptic function (Fox and Zinn, 2005; Johnson et al., 2006). Thus, based on these previous studies, two groups recently studied potential interactions between RPTPs and CSPGs/HSPGs and identified PTPσ and LAR as the critical receptors of CSPGs (Fisher et al., 2011; Sharma et al., 2012; Shen et al., 2009).
PTPσ inhibits neuronal growth as a CSPG receptor: Early studies indicated that the GAGs of HSPGs agrin and collagen XVIII interacted with the first Ig- like domain of PTPσ with high affinity and that PTPσ is able to regulate neuronal elongation (Aricescu et al., 2002; Rashid-Doubell et al., 2002), suggesting functional link of this RPTP with some sulfate proteoglycans. Given structural and functional similarity between HSPGs and CSPGs, the GAGs of CSPGs may also interact with PTPσ. A report indicates that PTPσ functions as one of the functional receptors for CSPGs (Shen et al., 2009). In vitro binding experiments support that the GAG chains of CSPG neurocan binds the first Ig-like domain of PTPσ through a number of positively-charged amino acids (Aricescu et al., 2002; Shen et al., 2009). Dorsal Root Ganglion (DRG) neurons cultured from PTPσ -/- mice had enhanced neurite growth on CSPG substrate, but not on Myelin Associated Glycoprotein (MAG), a myelin associated axon growth inhibitor. in vivo study with PTPσ -/- mice showed regrowth of lesioned ascending sensory axons in the fasciculus gracilis into CSPG-rich scar tissues (Shen et al., 2009). Consistently, a separate group detected regrowth of Corticospinal Tract (CST) axons into the caudal spinal cord in adult PTPσ deletion mice with T9 hemisection (Fry et al., 2010). Moreover, PTPσ deletion has been reported to stimulate regeneration of lesioned optic and peripheral nerves (Fry et al., 2010; McLean et al., 2002; Sapieha et al., 2005; Thompson et al., 2003). Therefore, a number of studies support that PTPσ is a functional receptor that partially mediates CSPG inhibition of neuronal growth.
LAR functions as a receptor for CSPGs to inhibit axon regeneration: Similar to PTPσ, LAR also interacts with the GAG chains of HSPGs with high affinity and regulates neuronal functions (Fox and Zinn, 2005; Johnson et al., 2006), including neurite outgrowth in vitro and nerve regeneration (Stepanek et al., 2005; Sun et al., 2000; Wang and Bixby, 1999; Wills et al., 1999; Xie et al., 2001; Yang et al., 2003; Yang et al., 2005; Yang et al., 2006). We recently studied potential role of LAR phosphatase in regulating axon growth and identified it as another transmembrane receptor of CSPGs (Fisher et al., 2011). LAR is widely expressed in the adult brain and spinal cord, including neuronal soma and axon cylinders. LAR binds a mixture of purified CSPGs with high affinity and its first Ig-like domain is critical for CSPG-LAR interactions also through GAG chains of CSPGs. CSPG treatment increases activity of LAR phosphatase in vitro. Importantly, inhibition of LAR by protein deletion or sequence-targeting blocking peptides partly enhanced neurite outgrowth of DRG cultures on CSPG substrate (Fisher et al., 2011), but not CNS myelin inhibitors. Thus, LAR activation due to selective CSPG stimulation in part suppresses extension of neurons. The remaining inhibition by CSPGs after LAR inhibition is probably mediated by other receptors and/or receptor-independent mechanisms (Carulli et al., 2005; Kwok et al., 2011; Shen et al., 2009).
We studied in vivo role of LAR in restricting regrowth of injured descending CNS axons with both transgenic and pharmacological approaches. LAR protein was upregulated days to weeks after injury and co-localized to various projection tracts, including serotonergic and CST axons (Fisher et al., 2011; Xu et al., 2015). LAR deletion increased regrowth of serotonergic axons into the scar tissues and caudal spinal cord after dorsal over-hemitransection at T7. LAR deletion also enhanced regrowth of CST fibers into the caudal spinal cord and improved functional recovery by increasing Basso mouse scale locomotor scores and stride length and reducing grid walk errors. Thus, LAR plays a crucial role in restricting regrowth of injured CNS axons. Moreover, pharmacological LAR blockade with sequence- targeting peptides stimulates regrowth of descending axons and recovery of locomotor function (Fisher et al., 2011).
Further recent studies also support the role of PTPσ and LAR in mediating CSPG function in the CNS. Newly-generated neurons from neuronal restricted precursors express low levels of PTPσ and LAR proteins and are intrinsically insensitive to CSPG substrates. Secreted factors by cultured neuronal and glial restricted precursors reduce CSPG inhibition and promote axonal growth in vitro (Ketschek et al., 2012). CSPGs could reduce growth, attachment, survival, proliferation of neural progenitor cells and differentiation of oligodendrocytes through activation of PTPσ and LAR (Dyck et al., 2015). Lamprey, a type of jawless fish, has heterogeneous neuronal regeneration capabilities after CNS injury and only some descending reticulospinal neurons regenerate after SCI. CSPGs are upregulated in the lesioned spinal cord of lamprey. Both PTPσ and LAR are selectively expressed in bad-regenerating neurons and have overlapping cellular distributions, indicating likely connection between activation of CSPG receptors and poor intrinsic regenerative ability of bad-regenerating neurons in non-mammals (Zhang et al., 2013).
Additional receptors that might mediate CSPG function
NgR1 is known as the receptor for three myelin inhibitors Nogo, MAG and oligodendrocyte myelin glycoprotein (Fournier et al., 2001; Fournier et al., 2002; Liu et al., 2006; McGee and Strittmatter, 2003). Further studies led to identification of the NgR homologs NgR2 and NgR3 (Lauren et al., 2003; Lauren et al., 2007). NgRs are GPI-linked membrane proteins and have similar structures, including eight Leucine-Rich Repeats (LRRs) flanked by N-terminal and C-terminal LRR-capping domains. NgR2 could bind MAG (Venkatesh et al., 2005), but ligands for NgR3 are not known. NgR1 and NgR3 have been shown to interact with CSPGs and mediate neuronal growth inhibition (Dickendesher et al., 2012). Deletion of both NgR1 and NgR3 in double knockout mice in part overcame CSPG inhibition and increased regeneration of crushed optic nerve axons, suggesting that NgR1 and NgR3 function as additional CSPG receptors. Also, the versican-NgR2 interactions appear to mediate plasticity of peripheral sensory fibers at dermo-epidermal junctions (Baumer et al., 2014). NgR2 specifically interacts with C-terminal G3 domain of versican and NgR2 deficient nociceptive nonpeptidergic sensory neurons was less sensitive to inhibition by skin-derived versican.
Out of 3 RPTPs in the LAR subfamily, PTPσ and LAR are known to convey CSPG inhibition as functional receptors. It will be interesting to determine whether PTPd also severs as a CSPG receptor.
Lecticans were usually employed to study CSPG-receptor interactions. Both PTPσ and LAR could interact with HSPGs as well as CSPGs and regulate their function (Coles et al., 2011; Wang et al., 2012). It is possible that the CSPGs phosphacan and NG2 and other sulfate proteoglycans (HSPGs and KSPGs) share the same and/or employ distinct receptors with lecticans. For example, chondroitin sulphate 4, 6 polysaccharides interact with the cell adhesion molecule contactin-1 (a GPI- anchored neuronal membrane protein) in neuroblastoma cell line and primary hippocampal neurons and modulate neurite outgrowth (Mikami et al., 2009). It is also interesting to determine whether contactin-1 functions as a receptor for CSPGs or other proteoglycans to regulate axonal growth.
Intracellular pathways that may mediate function of CSPGs and their receptors
Several intracellular signals have been implicated to convey CSPG suppression on neuronal growth, including RhoA, Akt, Glycogen Synthase Kinase 3β (GSK-3β), Protein Kinase C (PKC) and other signals (Figure 1) (Dill et al., 2008; Fu et al., 2007; Monnier et al., 2003; Powell et al., 2001; Sivasankaran et al., 2004). The signaling pathways that are downstream of CSPG receptors PTPs and LAR to mediate neuron growth failure have not been well studies. By using cerebellar granule neuronal cultures from postnatal LAR knockout mice and measuring activities a few signaling proteins, we found that activation of RhoA and inactivation of Akt signals regulate CSPGLAR interactions on growth inhibition. CSPG application increased the levels of active RhoA and decreased the levels of phosphorylated Akt at Ser473 in neurons derived from wild-type mice, but not in LAR -/- neurons. In contrast, CSPG administration did not change the levels of phosphorylated Collapsin Response Mediator Protein 2 (CRMP2) at Thr514 in either LAR +/+ or -/- neurons, indicating minimal role of CRMP2 in mediating LAR action due to CSPG stimulation. Similarly, activation of RhoA pathway and inactivation of Akt and TrkB signals appear to mediate PTPσ suppression on axon growth and dendritic spine formation (Kurihara D, 2012). Other signals, such as Erk signaling, may also convey the CSPG-receptor interactions because application of a LAR peptide and deletion of PTPσ increased activities of both Erk and Akt in neurons (Sapieha et al., 2005; Xie et al., 2006). Together, PTPσ and LAR at least share certain pathways to mediate CSPG function.
Local translation of RhoA in axons probably contributes to CSPG inhibition (Walker et al., 2012). Axons of cultured DRGs contain transcripts encoding RhoA and application of CSPGs to axonal compartment augmented intra-axonal RhoA synthesis. Accordingly, reduction of RhoA transcripts in axons promoted their growth in the presence of CSPGs. As an ATP-dependent motor protein, Myosin II probably also mediate CSPG inhibition on neuronal growth (Hur et al., 2011; Yu et al., 2012). CSPGs elevated phosphorylation of nonmuscle myosin II regulatory light chains and suppression of myosin II by a pharmacological or genetic approach enhanced axon growth on inhibitory substrates including CSPGs. NG2 has been shown to block axon growth by increasing the activities of PKC? and Cdc42 through PKC?-Par6 interactions (Lee et al., 2013). Of note, scar-sourced growth inhibitors share certain downstream signals with other repulsive molecules (such as myelin associated inhibitors) to regulate neuronal growth, including activation of RhoA and inactivation of Akt (Dill et al., 2008; Dill et al., 2010; Etienne-Manneville and Hall, 2002; Fisher et al., 2011; Fu et al., 2007; Luo, 2000; McGee and Strittmatter, 2003; Mueller et al., 2005).
Promote CNS axon regeneration by targeting CSPGs and receptors
It is very important to surmount the scar-mediated inhibition around lesion for achieving functional recovery after CNS injuries. The current major in vivo method to overcome CSPG inhibition is digest GAGs of CSPGs with locally applied bacterial enzyme ChABC. Because a few disadvantages may prevent using this enzyme as a therapeutic option for patients, it is critical to identify novel approaches to block CSPG function. Identification of CSPG receptors and better understanding of the signaling pathways activated by CSPGs may facilitate development of effective therapies to promote neural repair and functional recovery after CNS injuries.
Enhance neuronal growth by digesting CSPGs with ChABC
Treatment with ChABC in vitro could remove up to 88% of sulfated GAGs of CSPGs (Henninger et al., 2010) and remarkably enhance neurite outgrowth in neurons cultured on CSPG substrates (Busch et al., 2009; Kigerl et al., 2009). Local administration of ChABC to injured CNS in vivo has been widely employed to promote regeneration of lesioned axons and collateral sprouting of spared axons (Bradbury et al., 2002; Crespo et al., 2007; Fawcett, 2006; Jefferson et al., 2011). So far, ChABC application has been reported to promote regrowth of axons and formation of synaptic contacts along a number of axonal pathways, including corticospinal, serotoninergic, reticulospinal, nigrostriatal, ascending sensory axons and Clarke’s nucleus neurons (Barritt et al., 2006; Bradbury et al., 2002; Fouad et al., 2005; Garcia-Alias et al., 2009; Garcia-Alias et al., 2011; Moon et al., 2001; Tom et al., 2009; Yick et al., 2000). Transgenic expression of ChABC in reactive astrocytes has also been shown to promote regrowth of lesioned descending CSTs and ascending sensory fibers in the spinal cord (Cafferty et al., 2007).
ChABC treatment exhibits additive effects when combined with other regenerative strategies, including transplants of different types of cells or biomaterials, applications of neurotrophic factors or compounds that block myelin inhibitors, and other effective approaches (Alilain et al., 2011; Bradbury and Carter, 2011; Chau et al., 2004; Crespo et al., 2007; Fouad et al., 2005; Garcia-Alias et al., 2009; Garcia-Alias et al., 2011; Houle et al., 2006; Ikegami et al., 2005; Mingorance et al., 2006; Tom et al., 2009). CSPG digestion by local ChABC at the edge of cell transplants could facilitate axon exit from the grafts into the spinal cord (Alilain et al., 2011; Fouad et al., 2005; Tom et al., 2009). Combined ChABC and nerve autograft resulted in longer regeneration of serotonin and other projection tracts and better recovery of functions after SCI. Transplanted Schwan cells genetically modified to secrete ChABC and a neurotrophin into subacutelycontused spinal cord in rats enhanced regrowth of multiple axonal tracts (propriospinal, CST, 5-HT and other brainstem projecting fibers) into and caudal to the grafts and the number of myelinated axons, thus promoting recovery of locomotor and sensory functions (Kanno et al., 2014).
Most investigators evaluated roles of ChABC treatment with axonal tracing and/or immunostaining in animal models of incomplete injuries. Because it is challenging to differentiate regenerating axons from sprouting of undamaged fibers, both axon regeneration of disconnected axons and sprouting from spared fibers probably contributed to enhanced behavioral recovery and plasticity in most studies. However, a small number of laboratories reported enhanced axon regeneration and functional recovery in adult rodents with complete transection injury following treatments with local ChABC and other strategies (Bai et al., 2010; Fouad et al., 2005; Fouad et al., 2009). Although digestion of GAG chains by ChABC is the major molecular basis to overcome CSPG function, this enzyme may facilitate recovery after CNS injuries through other mechanisms, such as upregulation of tissue plasminogen activator and plasmin, altered orientation of astrocytic processes to guide elongation of regenerating axons (Milbreta et al., 2014), activated M2 macrophages, remodelling of specific CSPGs, promoting deposits of laminin and enhancing vascularity around lesion (Bartus et al., 2014).
Promote axon regeneration by inhibiting CSPG receptors and downstream pathways
Local ChABC may surmounts CSPG inhibition and promotes axon growth, but it has a number of disadvantages, which prevent its use to patients (Ohtake and Li, 2014; Sharma et al., 2012). It is important to develop new strategies to overcome inhibition by CSPGs and to stimulate CNS axon regeneration. An alternative approach to surmount scar-mediated inhibition is to design novel compounds to block functions of CSPGs or their receptors PTPσ, LAR and NgRs. Peptide antagonists for each of these receptors could increase regrowth of descending raphespinal axon growth and promoted sustained locomotor recovery in adult rodents with SCI (Fisher et al., 2011; GrandPre et al., 2002; Lang et al., 2014; Li and Strittmatter, 2003). By targeting recently-identified LAR receptor, we studied in vivo significance of LAR inhibition on regeneration of lesioned spinal cord axons with two blocking peptides. Systemic application of the Extracellular LAR Peptide (ELP) or Intracellular LAR Peptide (ILP) increased the density of serotonergic axons in the spinal cord 5-7 mm caudal to dorsal over-hemitransection T7 (Fisher et al., 2011). Longitudinal sections containing the lesion demonstrate regrowth of many 5-HT-positive axons into the CSPG-rich scar tissues and caudal spinal cord in ELP/ILP-treated mice. Peptide treated mice also performed better behavioral recovery, including enhanced locomotor Basso Mouse Scale scores and reduced grid walk errors of the hind paws a few weeks after injury. Thus, LAR blockade a pharmacological approach improves axonal growth and behavioral recovery in adult rodents with SCI, suggesting its great therapeutic potential for CNS injuries.
PTPσ is another receptor for CSPG inhibitors and its deletion has shown to increase regeneration and sprouting after various neurological injuries (Lang et al., 2014). Given blocking specific domains for LAR promotes CNS axon regeneration, targeting the same region of the similar member of PTPσ is also likely to stimulate axon elongation by overcoming CSPG function. In collaborating with our lab, the Silver’s group systemically applied the Intracellular PTPσ Peptides (ISP) to adult rats with severe thoracic contusive SCI, a model mimicking lesions of many SCI patients. Subcutaneous injections of ISP for 7 weeks induced significant functional recovery of both locomotor and bladder systems, along with a high volume of restored serotonergic innervation to the caudal spinal cord below the level of the lesion. Thus, inhibition of PTPσ with a selective antagonist remarkably promotes axon regrowth and behavioral recovery after SCI.
Systemic application of RPTP peptides efficiently blocks CSPG function in contrast to the highly invasive approach of applying ChABC locally. Receptor blockade should also avoid the issues of incomplete digestion of CSPGs and digestion of other sulfated proteoglycans that have beneficial roles for recovery. The above peptide studies not only further validate the critical role of PTPσ/ LAR in mediating growth inhibition of neurons by CSPGs within the injured adult spinal cord, also demonstrate that the strong suppression by CSPGs following CNS injuries can be overcome by systemic delivery of sequence-targeting peptides in vivo. These results open a new therapeutic avenue in non-invasive treatments for enhancing functional recovery following a variety of injuries where highly sulfated proteoglycans in the scar or PNN halt the attempt of axons to regenerate. Given that multiple factors are responsible for neural repair failure after CNS injury, combining CSPG receptor blockade with other strategies, such as cell transplants, probably becomes more effective.
CSPGs and their receptors appear to use a number of intracellular signaling pathways to mediate suppression on neuronal growth, including activating the small GTP-binding signaling protein RhoA (Figure 1) (Luo, 2000; Mueller et al., 2005; Walker and Olson, 2005). An alternative to promote axon growth in the presence to CSPGs is to influence the common downstream pathways including RhoA and ROCK (Fu et al., 2007; Luo, 2000; Mueller et al., 2005). Pharmacological RhoA inhibition by C3 transferase and some nonsteroidal anti-inflammatory drugs stimulates axon growth, overcomes CSPG suppression and improves behavioral recovery in rodents with SCI (Dergham et al., 2002; Dill et al., 2010; Fournier et al., 2003; Fu et al., 2007; Xing et al., 2011). A phase I/IIa clinical trial of an inhibitor of RhoA has been completed, with results suggesting that the treatment is safe and possibly beneficial (Fehlings et al., 2011). GSK-3β signal also in part mediates CSPG function and its inhibitors overcome CSPG suppression of neuronal growth (Dill et al., 2008; Fisher et al., 2011). GSK-3β inhibitors, particularly the clinical drug lithium, have been reported to be beneficial after CNS injuries in vivo. Lithium has also been evaluated for treating chronic SCI patients in phase I/II clinical trials (Yang et al., 2012). Of note, blockade of an individual downstream signaling, such as RhoA, may overcome suppression by several extracellular molecules on neuronal growth because multiple inhibitors usually share the same intracellular pathways.
Overcome scar-mediated inhibition and promote axon regeneration by other alternative approaches
Other strategies have also been considered to stimulate axon regeneration by suppressing CSPG inhibition. Decorin application in vitro enhanced neurite growth on both CSPGs and myelin membranes (Minor et al., 2008). Decorin down-regulates levels of CSPGs and promotes axon regrowth after SCI (Ahmed et al., 2014; Davies et al., 2004; Minor et al., 2008; Minor et al., 2011). Knockdown of chondroitin polymerizing factor, a major synthetic enzyme for CSPG GAGs, with an siRNA, attenuates GAG generation and CSPG suppression (Laabs et al., 2007). Disrupting assembly of GAG chains by knocking down xylosyltransferase-1 with deoxyribozyme also blocks CSPG inhibition (Grimpe and Silver, 2004; Hurtado et al., 2008; Oudega et al., 2012). Reactive astrocytes after CNS injuries produce high levels of Old Astrocyte Specifically Induced Substance (OASIS), which upregulates Chondroitin 6-O- Sulfotransferase 1 (C6ST1), a major enzyme involved in CSPG sulfation (Okuda et al., 2014). Suppression of OASIS and C6ST1 might also attenuate CSPG sulfation and inhibition.
Additional approaches have been reported to block scar-mediated inhibition on neuronal growth. Deletion of four PNN components, including brevican, neurocan, tenascin-C and tenascin-R, in quadruple knockout mouse (Geissler et al., 2013), may further overcome scar-sourced inhibition. Overexpressing R-Ras GTPase, an upstream positive regulator of Phosphatidylinositide 3-Kinases (PI3K) signaling, stimulated growth cone elaboration and axon extension on CSPGs (Silver et al., 2014), suggesting that activating PI3K-Akt signals surmounts CSPG function. NG2 appears inhibitory and its blockade (such as with antibodies) may promote axon growth and recovery after CNS injury despite of the controversy on NG2 functions (Brown et al., 2012; Tan et al., 2006). Administration of Taxol, a mitotic inhibitor used for cancer chemotherapy clinically, reduced scar generation and enhanced serotonergic axon growth and functional recovery after SCI by suppressing transforming growth factor-β signaling (Hellal et al., 2011). Interferon gamma, a dimerized soluble cytokine, inhibited neurocan generation by activated astrocytes in vitro and enhanced the number of myelinated axons in contused spinal cord by upregulating glial cell-derived neurotrophic factor and insulin-like growth factor-1 as well as reducing neurocan accumulation around the lesion (Fujiyoshi et al., 2010).
Conclusion
Following CNS injuries, reactive glial scar tissues generate high levels of inhibitory molecules (particularly CSPGs) and form potent chemical and physical barriers to axon elongation. Although CSPGs may suppress neuronal growth by blocking functions of the ECM and cell adhesion molecule receptors (such as laminin and integrins), CSPGs have at least two PTP receptors and may also bind NgRs at the sites remote from the binding domains for myelin-associated inhibitors. Out of multiple axon growth inhibitors identified in the CNS, CSPGs are particularly vicious and form a formidable barrier to regeneration of lesioned axons. Identification of the CSPG receptors is not only important for better understanding scar-mediated suppression, but also for developing novel and successful therapies to promote neuronal regeneration. Importantly, recent identification of selective antagonists for CSPG receptors and subcutaneous injections of them initiated days after axonal lesions might provide a basis for achieving effective axonal regeneration and locomotor recovery in adult mammals with CNS axonal injuries given the obvious advantages of peptides over bacterial enzyme ChABC and the wide applications of FDA-approved peptide drugs in humans. Combinations of CSPG signaling blockade with other effective strategies, such as those to enhance intrinsic neuronal capability (Ohtake et al., 2014; Park et al., 2008), should become more effective to promote robust axon regeneration and functional recovery. However, many issues remain unknown regarding the receptor-mediated CSPG inhibition. Which receptor is most essential for mediating the scar inhibition? Are the expression and function of each CSPG receptor neuronal type dependent? Do the reported receptors completely convey inhibition by different CSPG molecules or is there other unidentified crucial receptor(s)? Does blockade of multiple receptors simultaneously have synergistic actions on promoting axon regeneration and functional recovery? How much of CSPG inhibition is accounted for by binding receptors vs. steric hindrance of ECM molecules? Do the CSPG receptors regulate functions of various glial cells in the CNS? Do these receptors employ the same or distinct downstream pathways to covey CSPG inhibition on neuronal growth? Further studies on these important issues may further facilitate development of therapies that maximally overcome scar-mediated suppression and promote robust regeneration after CNS injuries.
Acknowledgment
Supported by research grants to S.L. from NIH (1R01NS079432, 1R21NS066114 and 1R01EY024575) and Shriners Research Foundation (86300).
References
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 2010; 58: 857-869.
- Ahmed Z, Bansal D, Tizzard K, Surey S, Esmaeili M, Gonzalez AM, et al. Decorin blocks scarring and cystic cavitation in acute and induces scar dissolution in chronic spinal cord wounds. Neurobiol Dis. 2014; 64: 163-176.
- Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 2011; 475: 196-200.
- Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002; 22: 1881-1892.
- Bai F, Peng H, Etlinger JD, Zeman RJ. Partial functional recovery after complete spinal cord transection by combined chondroitinase and clenbuterol treatment. Pflugers Arch. 2010; 460: 657- 666.
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006; 26: 10856-10867.
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014; 34: 4822-4836.
- Bäumer BE, Kurz A, Borrie SC, Sickinger S, Dours-Zimmermann MT, Zimmermann DR, et al. Nogo receptor homolog NgR2 expressed in sensory DRG neurons controls epidermal innervation by interaction with Versican. J Neurosci. 2014; 34: 1633-1646.
- Bixby JL. Receptor tyrosine phosphatases in axon growth and guidance. Neuroreport. 2000; 11: 5-10.
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002; 416: 636-640.
- Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011; 84: 306-316.
- Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA. 2012; 109: 4768-4773.
- Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009; 29: 9967-9976.
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007; 27: 2176-2185.
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005; 15: 116-120.
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004; 18: 194-196.
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, et al. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011; 332: 484-488.
- Condic ML, Snow DM, Letourneau PC. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J Neurosci. 1999; 19: 10036-10043.
- Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007; 206: 159-171.
- Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004; 19: 1226-1242.
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005; 29: 40-55.
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006; 281: 17789-17800.
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002; 22: 6570-6577.
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012; 15: 703-712.
- Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008; 28: 8914-8928.
- Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci. 2010; 30: 963-972.
- Dyck SM, Alizadeh A, Santhosh KT, Proulx EH, Wu CL, Karimi-Abdolrezaee S. Chondroitin Sulfate Proteoglycans Negatively Modulate Spinal Cord Neural Precursor Cells by Signaling through LAR and RPTPsigma and modulation of the Rho/ROCK Pathway. Stem Cells. 2015.
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002; 420: 629-635.
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004; 24: 2143-2155.
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006; 23: 371-383.
- Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011; 28: 787-796.
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, et al. Leukocyte Common Antigen-Related Phosphatase Is a Functional Receptor for Chondroitin Sulfate Proteoglycan Axon Growth Inhibitors. J Neurosci. 2011; 31: 14051-14066.
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005; 25: 1169-1178.
- Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord. 2009; 47: 727-732.
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001; 409: 341-346.
- Fournier AE, GrandPré T, Gould G, Wang X, Strittmatter SM. Nogo and the Nogo-66 receptor. Prog Brain Res. 2002; 137: 361-369.
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003; 23: 1416-1423.
- Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol. 2005; 15: 1701-1711.
- Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010; 58, 423-433.
- Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007; 27: 4154-4164.
- Fujiyoshi T, Kubo T, Chan CC, Koda M, Okawa A, Takahashi K, et al. Interferon-gamma decreases chondroitin sulfate proteoglycan expression and enhances hindlimb function after spinal cord injury in mice. J Neurotrauma. 2010; 27: 2283-2294.
- García-Alías G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009; 12: 1145-1151.
- Garcia-Alias G, Petrosyan HA, Schnell L, Horner PJ, Bowers WJ, Mendell LM, et al. Chondroitinase ABC combined with neurotrophin NT-3 secretion and NR2D expression promotes axonal plasticity and functional recovery in rats with lateral hemisection of the spinal cord. J Neurosci. 2011; 31: 17788-17799.
- Geissler M, Gottschling C, Aguado A, Rauch U, Wetzel CH, Hatt H, et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci. 2013; 33: 7742-7755.
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005; 29: 545-558.
- GrandPré T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002; 417: 547-551.
- Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci. 2004; 24: 1393-1397.
- Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, et al. The unusual response of serotonergic neurons after CNS Injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J Neurosci. 2011; 31: 5605-5616.
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011; 331: 928-931.
- Henninger HB, Underwood CJ, Ateshian GA, Weiss JA. Effect of sulfated glycosaminoglycan digestion on the transverse permeability of medial collateral ligament. J Biomech. 2010; 43: 2567-2573.
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006; 26: 7405-7415.
- Hrabetova S, Masri D, Tao L, Xiao F, Nicholson C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol. 2009; 587: 4029-4049.
- Hur EM, Yang IH, Kim DH, Byun J, Saijilafu, Xu WL, et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci USA. 2011; 108: 5057-5062.
- Hurtado A, Podinin H, Oudega M, Grimpe B. Deoxyribozyme-mediated knockdown of xylosyltransferase-1 mRNA promotes axon growth in the adult rat spinal cord. Brain. 2008; 131: 2596-2605.
- Ikegami T, Nakamura M, Yamane J, Katoh H, Okada S, Iwanami A, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci. 2005; 22: 3036-3046.
- Jefferson SC, Tester NJ, Howland DR. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J Neurosci. 2011; 31: 5710-5720.
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006; 49: 517-531.
- Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, et al. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci. 2014; 34: 1838-1855.
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004; 44: 961-975.
- Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol. 2012; 46: 251-264.
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012; 235: 627-637.
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009; 29: 13435-13444.
- Kolkman MJ, Streijger F, Linkels M, Bloemen M, Heeren DJ, Hendriks WJ, et al. Mice lacking leukocyte common antigen-related (LAR) protein tyrosine phosphatase domains demonstrate spatial learning impairment in the two-trial water maze and hyperactivity in multiple behavioural tests. Behav Brain Res. 2004; 154, 171-182.
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011; 71: 1073-1089.
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007; 27: 14494-14501.
- Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, et al. Modulation of the proteoglycan receptor PTPÏ promotes recovery after spinal cord injury. Nature. 2015; 518: 404-408.
- Laurén J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Mol Cell Neurosci. 2003; 24: 581-594.
- Laurén J, Hu F, Chin J, Liao J, Airaksinen MS, Strittmatter SM. Characterization of myelin ligand complexes with neuronal Nogo-66 receptor family members. J Biol Chem. 2007; 282: 5715-5725.
- Ledig MM, Haj F, Bixby JL, Stoker AW, Mueller BK. The receptor tyrosine phosphatase CRYPalpha promotes intraretinal axon growth. J Cell Biol. 1999; 147: 375-388.
- Lee SI, Zhang W, Ravi M, Weschenfelder M, Bastmeyer M, Levine JM. Atypical protein kinase C and Par3 are required for proteoglycan-induced axon growth inhibition. J Neurosci. 2013; 33: 2541-2554.
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003; 23: 4219-4227.
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006; 361: 1593-1610.
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000; 1: 173-180.
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003; 26: 193-198.
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991; 11: 3398-3411.
- McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knock- out mice. J Neurosci. 2002; 22: 5481-5491.
- Meathrel K, Adamek T, Batt J, Rotin D, Doering LC. Protein tyrosine phosphatase sigma- deficient mice show aberrant cytoarchitecture and structural abnormalities in the central nervous system. J Neurosci Res. 2002; 70: 24-35.
- Mikami T, Yasunaga D, Kitagawa H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J Biol Chem. 2009; 284: 4494-4499.
- Milbreta U, von Boxberg Y, Mailly P, Nothias F, Soares S. Astrocytic and vascular remodeling in the injured adult rat spinal cord after chondroitinase ABC treatment. J Neurotrauma. 2014; 31: 803-818.
- Mingorance A, Solé M, Munetón V, Martínez A, Nieto-Sampedro M, Soriano E, et al. Regeneration of lesioned entorhino-hippocampal axons in vitro by combined degradation of inhibitory proteoglycans and blockade of Nogo-66/NgR signaling. FASEB J. 2006; 20: 491-493.
- Minor K, Tang X, Kahrilas G, Archibald SJ, Davies JE, Davies SJ. Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol Dis. 2008; 32: 88-95.
- Minor KH, Bournat JC, Toscano N, Giger RJ, Davies SJ. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011; 134: 1140-1155.
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003; 22: 319-330.
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001; 4: 465-466.
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005; 4: 387-398.
- Ohtake Y, Li S. Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res. 2014.
- Ohtake Y, Park D, Abdul-Muneer PM, Li H, Xu B, Sharma K, et al. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014; 35: 4610-4626.
- Okuda H, Tatsumi K, Horii-Hayashi N, Morita S, Okuda-Yamamoto A, Imaizumi K, et al. OASIS regulates chondroitin 6-O-sulfotransferase 1 gene transcription in the injured adult mouse cerebral cortex. J Neurochem. 2014; 130: 612-625.
- Oohira A, Matsui F, Katoh-Semba R. Inhibitory effects of brain chondroitin sulfate proteoglycans on neurite outgrowth from PC12D cells. J Neurosci. 1991; 11: 822-827.
- Oudega M, Chao OY, Avison DL, Bronson RT, Buchser WJ, Hurtado A, et al. Systemic administration of a deoxyribozyme to xylosyltransferase-1 mRNA promotes recovery after a spinal cord contusion injury. Exp Neurol. 2012; 237: 170-179.
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008; 322: 963-966.
- Powell EM, Mercado ML, Calle-Patino Y, Geller HM. Protein kinase C mediates neurite guidance at an astrocyte boundary. Glia. 2001; 33: 288-297.
- Rashid-Doubell F, McKinnell I, Aricescu AR, Sajnani G, Stoker A. Chick PTPsigma regulates the targeting of retinal axons within the optic tectum. J Neurosci. 2002; 22: 5024-5033.
- Reinhard J, Horvat-Brocker A, Illes S, Zaremba A, Knyazev P, Ullrich A, et al. Protein tyrosine phosphatases expression during development of mouse superior colliculus. Exp Brain Res. 2009; 199: 279-297.
- Sapieha PS, Duplan L, Uetani N, Joly S, Tremblay ML, Kennedy TE, et al. Receptor protein tyrosine phosphatase sigma inhibits axon regrowth in the adult injured CNS. Mol Cell Neurosci. 2005; 28: 625-635.
- Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012; 237: 370-378.
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009; 326: 592-596.
- Sherman LS, Back SA. A 'GAG' reflex prevents repair of the damaged CNS. Trends Neurosci. 2008; 31: 44-52.
- Silver L, Michael JV, Goldfinger LE, Gallo G. Activation of PI3K and R-Ras signaling promotes the extension of sensory axons on inhibitory chondroitin sulfate proteoglycans. Dev Neurobiol. 2014; 74: 918-933.
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004; 7: 261-268.
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990; 109: 111-130.
- Snow DM, Watanabe M, Letourneau PC, Silver J. A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development. 1991; 113: 1473-1485.
- Snow DM, Smith JD, Gurwell JA. Binding characteristics of chondroitin sulfate proteoglycans and laminin-1, and correlative neurite outgrowth behaviors in a standard tissue culture choice assay. J Neurobiol. 2002; 51: 285-301.
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009; 32: 638-647.
- Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005; 25: 3813-3823.
- Stoker AW. Receptor tyrosine phosphatases in axon growth and guidance. Curr Opin Neurobiol. 2001; 11: 95-102.
- Sun Q, Bahri S, Schmid A, Chia W, Zinn K. Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. Development. 2000; 127: 801-812.
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006; 26: 4729-4739.
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011; 31: 6289-6295.
- Tan CL, Andrews MR, Kwok JC, Heintz TG, Gumy LF, Fässler R, et al. Kindlin-1 enhances axon growth on inhibitory chondroitin sulfate proteoglycans and promotes sensory axon regeneration. J Neurosci. 2012; 32: 7325-7335.
- Thompson KM, Uetani N, Manitt C, Elchebly M, Tremblay ML, Kennedy TE. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol Cell Neurosci. 2003; 23: 681-692.
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009; 29: 14881-14890.
- Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, et al. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000; 19: 2775-2785.
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006; 26: 5872-5880.
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, et al. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005; 25: 808-822.
- Walker BA, Ji SJ, Jaffrey SR. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J Neurosci. 2012; 32: 14442-14447.
- Walker K, Olson MF. Targeting Ras and Rho GTPases as opportunities for cancer therapeutics. Curr Opin Genet Dev. 2005; 15: 62-68.
- Wang F, Wolfson SN, Gharib A, Sagasti A. LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr Biol. 2012; 22: 373-382.
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, et al. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008; 121: 3083-3091.
- Wang J, Bixby JL. Receptor tyrosine phosphatase-delta is a homophilic, neurite-promoting cell adhesion molecular for CNS neurons. Mol Cell Neurosci. 1999; 14: 370-384.
- Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999; 22: 301-312.
- Xie Y, Yeo TT, Zhang C, Yang T, Tisi MA, Massa SM, et al. The leukocyte common antigen-related protein tyrosine phosphatase receptor regulates regenerative neurite outgrowth in vivo. J Neurosci. 2001; 21: 5130-5138.
- Xie Y, Massa SM, Ensslen-Craig SE, Major DL, Yang T, Tisi MA, et al. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J Biol Chem. 2006; 281: 16482-16492.
- Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, et al. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol. 2011; 231: 247-260.
- Xu B, Park D, Ohtake Y, Li H, Hayat U, Liu J, et al. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol Dis. 2015; 73: 36-48.
- Yang ML, Li JJ, So KF, Chen JY, Cheng WS, Wu J, et al. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: a double-blind, randomized, placebo-controlled clinical trial. Spinal Cord. 2012; 50: 141-146.
- Yang T, Bernabeu R, Xie Y, Zhang JS, Massa SM, Rempel HC, et al. Leukocyte antigen-related protein tyrosine phosphatase receptor: a small ectodomain isoform functions as a homophilic ligand and promotes neurite outgrowth. J Neurosci. 2003; 23: 3353-3363.
- Yang T, Yin W, Derevyanny VD, Moore LA, Longo FM. Identification of an ectodomain within the LAR protein tyrosine phosphatase receptor that binds homophilically and activates signalling pathways promoting neurite outgrowth. Eur J Neurosci. 2005; 22: 2159-2170.
- Yang T, Massa SM, Longo FM. LAR protein tyrosine phosphatase receptor associates with TrkB and modulates neurotrophic signaling pathways. J Neurobiol. 2006; 66: 1420-1436.
- Yeo TT, Yang T, Massa SM, Zhang JS, Honkaniemi J, Butcher LL, et al. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J Neurosci Res. 1997; 47: 348-360.
- Yick LW, Wu W, So KF, Yip HK, Shum DK. Chondroitinase ABC promotes axonal regeneration of Clarke's neurons after spinal cord injury. Neuroreport. 2000; 11: 1063-1067.
- Yu P, Santiago LY, Katagiri Y, Geller HM. Myosin II activity regulates neurite outgrowth and guidance in response to chondroitin sulfate proteoglycans. J Neurochem. 2012; 120: 1117-1128.
- Zhang G, Hu J, Li S, Huang L, Selzer ME. Selective expression of CSPG receptors PTPs and LAR in poorly regenerating reticulospinal neurons of lamprey. J Comp Neurol. 2014; 522: 2209-2229.