
Case Report
Austin J Neurol Disord Epilepsy. 2015; 2(1): 1011.
Alleviating Visual Impairment from Radiation-Induced Optic Neuropathy by Mouse Nerve Growth Factor
Xiaoming Rong, Focai Lin and Yamei Tang*
Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
*Corresponding author: Yamei Tang, Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong Province, China
Received: September 11, 2015; Accepted: October 02, 2015; Published: October 05, 2015
Abstract
We reported a 74-year old man who was diagnosed as radiation-induced optic neuropathy eight years after radiotherapy. Even though the visual loss has lasted for more than six months and did not response to corticosteroid monotherapy, his symptoms were improved after receiving mouse nerve growth factor plus methylprednisolone. This case report suggests that mouse nerve growth factor might be a promising treatment for radiation-induced optic neuropathy.
Keywords: Radiation-Induced Optic Neuropathy; Visual Impairment; Mouse Nerve Growth Factor
Introduction
Radiation-Induced Optic Neuropathy (RON) is a late complication of radiotherapy which typically occurs several months to years (average 18 months) after completion of radiotherapy [1]. It is presumably caused by radiation-induced damage to vascular endothelium and neuro glial cell progenitors [2,3]. Up till now, therapeutic options for RON include corticosteroid, anticoagulants, and Hyperbaric Oxygen (HBO). However, corticosteroid does not always work. Even anticoagulation and HBO also show inconsistent effect on RON [4-6]. Here we reported a case with RON whose visual impairment was greatly improved by using mouse nerve growth factor. This study may give a highlight for mouse nerve growth factor in treating RON.
Case Report
A 74-year-old man, who was diagnosed with nasopharyngeal carcinoma and received radiotherapy eight years ago, complained of progressive bilateral visual loss in the last 6 months. He was in generally good health until presenting at 6 months ago with blurred vision of the right eye. He denied eye pain, headache, nausea, vomiting, limb weakness, dysphagia or seizure. No medical history for diabetes mellitus, hypertension, and coronary arterial disease were reported. Unfortunately, his symptoms continued to develop and he noted that he could not see out of the right eye. He was first seen in other hospital and was diagnosed with RON. The patient was treated with oral prednisolone (40mg/day). However, the symptoms did not improve, instead, his right vision came to full black out and the left vision became worse and worse. The patient thus searched for second opinion in our hospital. When in admission, he was alert and attentive with no light perception in the right eye and just light perception in the left eye but full strength throughout. Physical examination revealed bilateral mild enlarged round pupils and sluggish direst response to light. No other notable neurologic deficiency was detected.
A cranial MRI with gadolinium contrast revealed small ischemic changes in the deep white matter without hemorrhage or typical radiation-induced brain necrosis (Figure 1). The signal of optic nerve was normal. Lumber puncture did not find abnormalities in cerebrospinal fluid (white cells 5*10^6/L, red cell 0*10^6/L, protein 0.38 g/L, RPR negative). Visual Evoked Potential (VEP) failed to recognize apparent P100 wave at both eyes (Figure 2A). Visual acuity/ visual field examination demonstrated diffuse visual loss bilaterally (Figure 3). And funduscopic examination found that the both side optic disc were pale. We also checked routine blood tests as well as thyroid function which were all negative or within normal range.
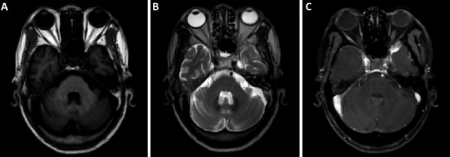
Figure 1: Cranial MRI for the 74-year old man with RON. (A) Axial, T1-
weighted image, (B) Axial, T2-weighted image and (C) T1-weighted image
with gadolinium contrast enhancement. The images showed normal signal in
both optic nerves and no typical radiation-induced brain injury was noticed.
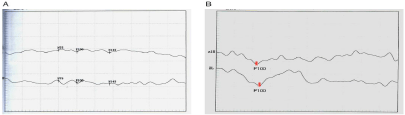
Figure 2: Visual evoked potential before and after treatment. (A) No apparent
P100 was detected in both eyes. (B) Six months after treatment, the left eye
showed reappeared P100 wave.
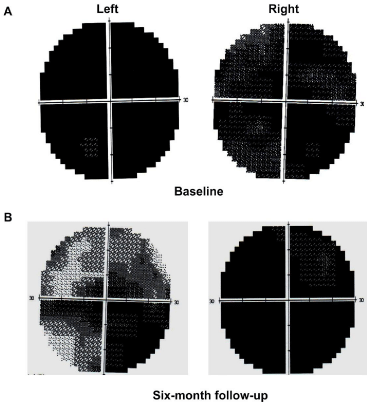
Figure : Visual acuity/visual field examination before and after treatment. At
baseline, diffuse visual loss was noticed bilaterally. After six-month treatment,
the visual acuity was improved in left eye, while the right eye did not show
significant improvement.
With regards to the radiotherapy history, progressive diffuse painless vision loss in both eyes over weeks, VEP finding as well as neuroimaging result, late RON was diagnosed. Considering the unsatisfied response to corticosteroid in other hospital, we used mouse nerve growth factors (mNGF; Enjingfu, Xiamen Beida Road Bioenineering, China). 9000 Au mNGF was dissolved in 2 ml normal saline and injected intramuscularly at 18μg/time, once a day for consecutive two weeks. Meanwhile, corticosteroid therapy was adapted to methylprednisolone 80mg per day intravenously for five days and followed by methylprednisolone 40mg per day for 3 days and then oral prednisolone 30mg per day. Each treatment cycle was about two weeks. His visual symptoms were gradually improved early at the second week of treatment. The patients then received repeated treatment with mNGF plus corticosteroid four times in six months and his symptoms became stable. A follow-up VEP at six months showed reappeared P100 wave in the left eye (Figure 2B). There was also improvement in visual acuity that he could recognize hand movement with left eye, although the visual acuity in the right eye did not improve well. An inform consent was obtained from this patient because mNGF is still not labeled for the use in RON.
Discussion
The diagnosis of RON is generally suspected from the clinical characteristics and may be confirmed in most cases with VEP, funduscopic examination and neuroimaging in the absence of other causes. Irreversible painless visual impairment (loss of visual acuity and/or visual field defect) and absence or low amplitude of P100 wave without other causes indicate the diagnosis of RON [7]. MRI with gadolinium in the acute phase of RON may reveal enhancement of the optic nerve, however, it is not a necessary criteria [8,9]. Differential diagnosis included infectious, post infectious and vascular-associated pathologies.
Conventional therapeutic options for RON include corticosteroid, anticoagulants, and HBO. However, HBO was only reported to be useful within 72 hour of visual loss, while corticosteroid and anticoagulants did not show consistent effect in previous studies [4,6]. Our patient showed no response to corticosteroid in other hospital but had improvement after being treated with corticosteroid plus mNGF indicated the potential role of mNGF on RON. Nerve growth factor is acknowledged as a protein that is important for the growth and maintenance of neurons. It is reported to be able to prevent apoptosis and degeneration of neurons [10]. Radiationassociated late complications such as brain necrosis and neuropathy are usually thought to be progressive and partially irreversible. Even though nerve growth factor is a large molecular that hardly cross normal blood brain barrier (BBB), endothelial cells injury and its subsequently increased permeability of BBB after radiotherapy enhance the capability of mNGF to cross BBB. The therapeutic effect of mNGF for radiation-induced brain necrosis has been reported in a small series report [11]. Our patient showed great improvement in vision from totally black out to count fingers two weeks after receiving treatment of mNGF and corticosteroid, which indicated the promising effect of mNGF on RON.
There are some limitations of this study, including small sample size and lack of comparison with similar NGF treatment. However, by comparing the therapeutic effect of the two different treatment strategies in the same patients, we thought that the effect of mNGF on RON could not be ignored [12]. It is still hard to make any definitive recommendation for RON by using mNGF after this case report. Further prospective studies with larger sample size are needed to prove the effect of mNGF on RON.
Acknowledgement
This work was supported by National Natural Science Foundation of China (No. 81072242 and No. 81272576) to Yamei Tang and the Medical Scientific Research Foundation of Guangdong Province (No. B2012100) to Xiaoming Rong.
References
- Danesh-Meyer HV. Radiation-induced optic neuropathy. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2008; 15: 95-100.
- Levin LA, Gragoudas ES, Lessell S. Endothelial cell loss in irradiated optic nerves. Ophthalmology. 2000; 107: 370-374.
- Myers R, Rogers MA, Hornsey S. A reappraisal of the roles of glial and vascular elements in the development of white matter necrosis in irradiated rat spinal cord. Br J Cancer Suppl. 1986; 7: 221-223.
- Borruat FX, Schatz NJ, Glaser JS, Feun LG, Matos L. Visual recovery from radiation-induced optic neuropathy. The role of hyperbaric oxygen therapy. J Clin Neuroophthalmol. 1993; 13: 98-101.
- Miller NR. Radiation-induced optic neuropathy: still no treatment. Clin. Experiment. Ophthalmol. 2004; 32: 233-235.
- Boschetti M, De Lucchi M, Giusti M, Spena C, Corallo G, Goglia U, et.al. Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing disease. Eur J Endocrinol. 2006; 154: 813-818.
- Zhao Z, Lan Y, Bai S, Shen J, Xiao S, Lv R, et.al. Late-onset radiation-induced optic neuropathy after radiotherapy for nasopharyngeal carcinoma. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2013; 20: 702-706.
- Zimmerman CF, Schatz NJ, Glaser JS. Magnetic resonance imaging of radiation optic neuropathy. Am J Ophthalmol. 1990; 110: 389-394.
- Guy J, Mancuso A, Beck R, Moster ML, Sedwick LA, Quisling RG, et.al. Radiation-induced optic neuropathy: a magnetic resonance imaging study. J Neurosurg. 1991; 74: 426-432.
- Luk YO, Chen WYK, Wong WJ, Hu HH, Hsu LC, Chern CM, et.al. Treatment of focal cerebral ischemia with liposomal nerve growth factor. Drug Deliv 2004; 11: 319-324.
- Wang X, Ying H, Zhou Z, Hu C, Eisbruch A. Successful treatment of radiation-induced temporal lobe necrosis with mouse nerve growth factor. J Clin. Oncol. 2011; 29:8-166.
- Levin VA, Bidaut L, Hou P, Kumar A J, Wefel JS, Bekele BN, et.al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys.2011; 79: 1487-1495.