
Research Article
Austin J Neurol Disord Epilepsy. 2016; 3(1): 1016.
Acquired German Accent: A Functional Neural Systems Approach to Foreign Accent Syndrome
Klineburger PC, Campbell RW, Harrison PK and Harrison DW*
Department of Psychology, Behavioral Neuroscience Laboratory, USA
*Corresponding author: David W Harrison, Department of Psychology, Behavioral Neuroscience Laboratory, Williams Hall, Virginia Tech, Blacksburg, VA, 24061-0436, USA
Received: November 11, 2015; Accepted: December 23, 2015; Published: January 27, 2016
Abstract
Foreign Accent Syndrome (FAS) is often acquired subsequent to stroke or traumatic brain injury, where the patient initially experiences muteness, aphasia, or apraxia, and then FAS during recovery and often before his/her normal speech pattern returns. Common areas for brain lesions in FAS are found around the left inferior frontal region, but lesion location varies among patients with this syndrome. Moreover, it appears that FAS may require combined lesions, with the respective lesion locations or lesion pattern relevant to the regional accent acquired. This case study presents the findings from a patient with an acquired and distinctly German accent following combined lesion of the left inferior frontal region and the pons. Although FAS has been described following a cerebellar lesion, the authors were not able to locate prior evidence in the literature of onset of FAS involving pontine cerebrovascular accident.
Keywords: Foreign accent syndrome; Lingual praxis; Prosody; Aphasia; Aprosodia; Speech; Speech disorders; Stroke; Cerebrovascular accident; Multiple infarct; Acquired speech disorder; Neuropsychology; Neurology; Pons; Brainstem; Cerebellum
Background
First described by French neurologist Pierre Marie in 1907, Foreign Accent Syndrome (FAS) is a rare speech disorders in which individuals take on, what is perceived by observers, to be a distinct accent differing in many respects from the individual’s native accent. FAS has been of great interest in the neuropsychological literature because its unique presenting features have challenged our understanding of the neural systems underlying the production of speech, shedding light on neural structures that had previously not been regarded as integral to speech production. In the approximately 60 reported cases of FAS, nearly all have involved a stroke or traumatic brain injury resulting in relatively small lesions of the language dominant hemisphere in the prerolandic motor cortex, insular cortex, the frontal motor association cortex, and/or the striatum of the language dominant hemisphere [1]. Several FAS cases with neuroimaging fingings have also shown lesions of the fronto-parietal regions, right hemisphere, basal ganglia, and the right cerebellum [2].
Despite a growing literature, there is current debate as to whether a common neural substrate gives rise to this syndrome and if FAS should be considered a distinct syndrome rather than a sub-type of Apraxia of Speech (AoS) [3]. It is also currently being debated whether or not individuals with FAS actually acquire a geographically distinct foreign accent, if FAS is in the “ear” of the beholder [4], or if there is perhaps a “generic” form of acquired foreign accent [5]. However, many agree that FAS is distinct from aphasic and Aprosodia disorders, per se. Indeed, due to the absence of componential speech deficits, FAS is considered to be categorically distinct from disorders of language or speech (aphasia, dysarthria, Aprosodia, etc.), and presents as normal such that observers interpret the deficit as one of a change in accent rather than an impediment of speech [6].
Individuals with FAS commonly present, in the initial stages following neurological insult, with aphasia symptoms that eventually diminish while FAS persists; do not have diminished fluency; do not exhibit speech altering errors with impaired comprehension or meaning; and often maintain a full range of prosody, rather than a diminished or exaggerated prosodic range. Relatively few cases in the FAS literature have provided details on lesion localization, further making it difficult to identify a clear-cut pattern of neuropathology in FAS. Blumstein and Kurowski [1] suggest that FAS emerges as a consequence of damage to speech output motor systems affecting the primary motor cortex and either cortico-cortical connections with these regions or its cortico-subcortical projections. Aside from cortical lesions of the anterior left hemisphere, lesions of subcortical areas such as the basal ganglia have also been commonly associated with FAS.
This exclusion of aphasic and dysarthria deficits as an explanation for FAS leaves the possibility that the disorder could be conceptualized as a form of apraxia of language or lingual apraxia [7].The apraxia are a broad category of disorders representing dysfunction of skilled motor movements, involving nearly any region of the body [8]. In this regard, lingual praxis follows from involvement of motor systems dedicated to both the planning and sequencing of speech output specific to the anterior regions of the left hemisphere, but also to those systems controlling pitch, intonation, and prosody specific to the homologous regions on the right.
In this case report, we present the investigation of an individual who acquired FAS after vascular infarcts affecting the inferior left frontal region as well as the left (acute) and right (remote) side of the brainstem at the level of the pons. FAS, in the present case, and following consideration of the literature, presented subsequent to the combined lesions, whereas the remote event was insufficient alone for the presenting features. The latter lesion site is the first to be reported in a case with FAS, although the involvement of hindbrain regions such as the right cerebellum has recently been reported [7]. Interestingly, the patient’s FAS symptoms wax and wane with arousal level, indicating a role of the brainstem arousal systems in FAS. This case study provides an opportunity to discuss FAS in the framework of Alexander Luria’s functional neural systems model.
Patient Information
The patient is a 70 year old right-handed female that suffered a stroke affecting the left inferior posterior frontal region and bilateral brainstem at the level of the pons. She is a retired service club administrator, now status post-acute left pontine infarct. The event appeared to result from vascular pathology rather than from and embolic event. Her medical record is remarkable for old right pontine infarct, old left frontal infarct, moderate white matter disease, a history of squamous cell carcinoma status post-resection, chronic atrial fibrillation status post-cardioversion, coronary artery disease with three stents, hypertension, hyperlipidemia, trochanteric bursitis status post-injection to the right, and a history of sarcoidosis involving the lungs and heart. Her bilateral carotids show less than 50% stenosis. Magnetic Resonance Imaging (MRI) revealed acute left pontine infarct, evidence of past right pontine infarct with a tiny area of encephalomalacia, and past inferior front parietal infarct.
Following her Cerebrovascular Accident (CVA), the patient acquired a distinct German accent, consistently identified by her multidisciplinary rehabilitation staff members, by her family, and by her friends. Furthermore, the patient perceived her own speech production as acquired, now reflecting a foreign German accent not heretofore experienced. Her foreign accent symptoms waxed and waned such that, with elevated arousal, the accent was pronounced. These episodes varied to periods of lowered arousal level, during which her speech was devoid of the German accent and more characteristic of her premorbid speech productions. At low levels of arousal, the patient’s speech volume would be dysarthria and, at high levels of arousal, the patient’s speech was louder and accompanied by a distinct German accent. She was evaluated and treated on a multidisciplinary rehabilitation ward at the medical center. She was referred for neuropsychological evaluation of functional cerebral systems, for participation in cognitive team planning and protocol development, and for provision of cognitive skills training and family education, per Social Services.
Observations
The patient’s affect was pleasant, although she expressed some discomfort in the acknowledgment of her acquired FAS. She also expressed appreciation of her deficits with some apprehension or fear related to the possibility of her next stroke. She presented as mildly dysphonic, but did improve somewhat with reassurance and with feedback on her standardized test battery performance in normative comparisons. She appeared to participate with good effort throughout the evaluation. Propositional speech content was somewhat hyper fluent at times. FAS symptoms waxed and waned correlating with her arousal level.
Tests Administered
The patient was administered a Neuropsychological Interview, Neurobehavioral Status Exam for Syndrome Analysis, Sensorimotor Screening, Affect Screening, Aphasia Screening, Screening for Spatial Awareness and Integration, the Dynamometer Grip Strength Test, the Geriatric Depression Scale (GDS), Luria’s Ramparts, selected figure copy tests, and related procedures. Neurocognitive evaluation was obtained using the Dementia Rating Scale - II (DRS-II) test battery. The latter scale includes normative performance comparisons across four subscales, including Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory. The Memory scale includes both visual and auditory verbal test measures. Also, the Initiation/Perseveration scale provides for standardized comparison on verbal and figural fluency measures.
Results
Affect screening
The patient presented with diminished energy, mild dysphoria, and apprehension related to her expectations for her next CVA. She confirmed her primary affective valence of sadness or dysphoria, but also with anger and fear concerns. Affect presentation also appeared dysphoric with diminished facial range and heightened zygomatic tone. The GDS confirmed some dysphoric complaints. The overall diagnostic impression from this component of the evaluation was for a mild depression or adjustment reaction at the time of evaluation. There were prior reports in the medical record, confirmed by the patient on interview, of insomnia associated with the pontine lesion history. However, the documentation of these complaints indicated that they appear to have been resolved with medication.
Aphasia screening
Speech presentation was complex with dysarthria and staccato features. Of note was that her symptoms of foreign accent (German) waxed and waned with her overall arousal level within and between sessions. She complained of receptive speech deficits and appreciated reading and writing deficits as clinical correlates of her left frontoparietal lesion on the MRI of the head (Figure 1). However, these functions remained intact for basic communication.
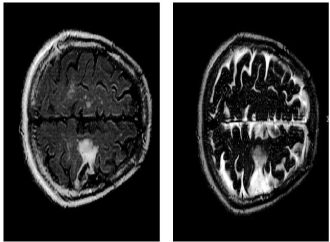
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Motor screening
Motor findings were complex with multiple lesions and waxing and waning of symptoms. Regardless, she presented with mild right hemiparesis. The right foot was frequently adducted. Parietal drift test showed down drift at the right arm. The Dynamometer Grip Strength Test showed right hand grip = 17 kg., whereas grip strength of 23kg was recorded at the left hand. There was also mild truncal ataxia with cerebellar or brainstem features and bilateral dysmetria worse at the right side. Ideomotor praxis at the distal upper extremities was intact. Her tongue deviated to the left side on extension (Figure 2).
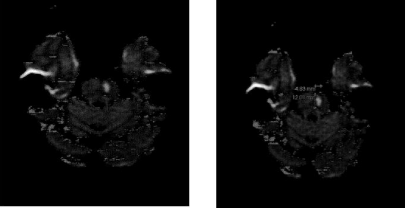
Figure 2: Bilateral pontine infarcts remote at the right and acute at the left
side of the pons. The image transects the upper pons.
Spatial awareness
She presented with significant right-sided proprioceptive deficits and a proprioceptive torticollis. She compensated for this with visual cues. Elimination of visual cues aggravated the proprioceptive deficits, remarkably. These deficits also waxed and waned across time. The patient’s left/right awareness and body part awareness were accurate on simple confrontation testing.
Sensory screening
She made errors in the localization of sound originating within either the left or the right hemispace. There was no extinction on dichotic testing. There was very mild nystagmus at the right eye on visual smooth pursuit. Her lateral and vertical gaze was full and persistent (Table 1).
Scale
Interpretation
Attention
Average
Initiation/ Perseveration
Mild Impairment
Construction
Intact
Conceptualization
Average
Memory
Intact
Overall
Intact Mild Cognitive Impairment
Table 1: Neurocognitive Assessment she completed the DRS-II test battery. The results are provided below for comparative purposes.
Overall performance reflected Mild Cognitive Impairment on standardized comparisons, largely secondary to mild performance deficits on measures of executive function on the Initiation/ Perseveration subscale of the DRS-II.
Patient Summary
The patient displayed a history of acute left pontine lesion, older right pontine lesion, and an old inferior left frontoparietal lesion. Standardized neurocognitive evaluation indicated intact cognitive ability in normative comparisons; consist with an overall interpretation of performance at the mild cognitive impairment range. She presented speech deficits consistent with foreign accent syndrome, with a characteristically German accent. However, the FAS waxed and waned over time, secondary to fluctuating arousal level. Dysarthria, staccato features, and a waxing and waning of receptive speech deficits, lexicon, and graphics were present. Motor deficits were present with right hemiparesis in addition to significant right sided proprioceptive deficits and proprioceptive torticollis, for which she compensated with the provision of visual cueing techniques.
Discussion
FAS might be better understood if approached from a functional cerebral systems approach incorporating the three principal functional units of the brain whose participation is necessary for the integrity and stability of neurocognitive functions [9]. From a functional cerebral systems approach, FAS can be thought of as a neuropsychological disorder resulting from damage to, and altered communication between, the three functional units to include the primary, secondary, and tertiary zones within each of the three units. The third functional unit, located in the regions anterior to the precentral gyrus [9] is responsible for programming, regulating, and verifying conscious activity such as speech planning, speech monitoring, and speech correction. Before speech production occurs, neural activity in the premotor region/secondary zone is systematically organized to coordinate movements such as forming vowels, consonants, words, speech patterns, and lingual rhythm. Speech organized in the premotor region is then passed through the structures of the primary motor cortex and ultimately to the vocal apparatus [9]. Many lesions in FAS spare the primary speech area (Broca’s area), and largely affect the secondary/premotor area in the left frontal association cortex. Herein lies the dissociation between Broca’s aphasia and FAS; the elemental units of speech production that are organized in the premotor region are affected resulting in the appearance of someone with a speech disorder but who is also verbally fluent and lacking the dysfunction of speech production. Because the premotor region of the brain regulating and altering elemental speech properties is dysfunctional, patients would be predicted to have difficulty trying to reorganize, correct, and resequence these smaller, more elemental speech properties, explaining why that even after intense speech therapy, FAS tends to persist while the co-occurring aphasia abates.
When the basal ganglia of the third functional unit is lesioned, as is commonly the case in FAS, the rhythm, intonation, pitch contours, volume, and overall delivery of speech are affected. Depending on other lesion sites involved and the size of the lesion in the basal ganglia, the above speech properties can be altered in a manner that contributes to a speech profile or accent similar to that of a foreign language. For example, the increased volume and staccato speech seen in basal ganglia lesioned patients may be perceived as a German or eastern European accent.
Motor speech symptoms resulting from cerebellar pathology are typically described as slow, monotonous, staccato, scanned, indistinct, remarkably irregular, jerky, explosive, slurred and laborious. These functional variants may prove useful in understanding FAS. The anatomical connections between the left cerebellum, sometimes referred to as the ‘lateralized’ linguistic cerebellum [7], and the motor speech centers of the language-dominant hemisphere, indicate that rather than a single lesion or disrupted neural site, it is the altered communication between these functional cerebral systems that contributes to FAS symptoms. Marien and Verhoeven [7] described a FAS case in which, subsequent to a 3 year follow up, resolution of FAS symptomology was found concurrent with neuroimaging results suggesting the improvement of a right cerebellar hypoperfusion. The authors provide evidence that FAS may result from altered functional connectivity between the subcortical brainstem mechanisms and those of the higher cortical areas.
To date, no cases of FAS have reported a lesion in the pontine area of the brainstem. In the current case study, the patient suffered a stroke in the right brainstem at the level of the pons as well as a perisylvian lesion, suggesting that FAS and speech production disorders can involve each of these three functional cerebral systems and their connections rather than a strictly localized lesion in the frontal areas. Additionally, arousal levels waxed and waned in this patient simultaneously with the waxing and waning of FAS symptoms such that during times of heightened arousal, FAS symptoms were most pronounced. This waxing and waning of arousal and FAS symptoms may provide evidence that dysregulation resulting from frontal lobe lesions, which normally act to adjust levels of arousal projecting from the reticular activating system in the brainstem, is another factor in the presentation of FAS. This interplay of functional cerebral regions and brainstem units and the variability of FAS symptoms show the reciprocal role of distal brain regions in FAS and may have implications for other motor speech disorders. Furthermore, the close proximity of the right pons lesion to the previously discussed right cerebellum further demonstrates the involvement of multiple functional units in producing this speech disorder.
Conceptualizing this particular case of FAS as a lingual apraxia with associated fronto-cerebellar-pontine arousal dysfunction may allow for explaining the waxing and waning of the acquired accent as well as the altered pitch in this patient. Specifically, the reciprocal connections via the frontodendatorubrothalamic relay provide for the intonation of speech with prosody. Right frontal lesions have historically been associated with changes in prosody and intonation of speech [8]. In this case, lesions result in expressive Aprosodia and a characteristic flat affect as indicated by the lack of inflection in the speech of the individual. In the case of FAS, a disruption to the aforementioned reciprocal connections between the frontal speechmotor systems and the brainstem mechanisms involved in motor speech planning (i.e. cerebellum) may result in the characteristic pitch changes seen in FAS. Research has suggested the close interaction between left anterior regions involved in motor-speech output and the cerebellum [10].
Although there is consistency in several lesion sites in FAS, researchers agree that no clear cut pattern of neuropathology can explain FAS. Evidence from neuroimaging studies now demonstrates the involvement of the three functional cerebral units in FAS [11] suggesting that a common neurophysiological lesion profile may not be necessary to adequately understand FAS. Rather, it may be that the specific combination of lesions in geographically disparate but anatomically and functionally related areas of the brain that mimics a specific foreign accent. If this is the case, then different ‘foreign’ accents could be expected depending on the lesion pattern(s) or locations.
Patients suffering from FAS have also reported a range of negative and positive emotional responses as a result of their newly acquired foreign accent, but investigations into the emotional aspects of FAS have not been systematically examined and in general, have been less well studied. Individuals acquiring FAS have reported that it has impacted their psychosocial interactions, hindering relationships for some while improving those of others. With the most typical lesion site in FAS involving the left hemisphere, depressed mood could be expected to co-occur with FAS as well as other symptoms such as lethargy, lack of motivation, and decreased initiation based on the valence theory of emotion [12]. These depressive symptoms are also likely exacerbated by social difficulties in approach related behavior and in emotional bias [13]. In one case, a saleswoman was not able to continue work due to her newly acquired, and ‘odd’, accent and another patient described feeling as if she had “lost a part of herself” after acquiring FAS. The sense of self and community associated with having a distinct regional accent or dialect that becomes disrupted after suddenly acquiring a foreign accent may occur and should be considered during assessment and therapy.
Conclusion
This case presents evidence of foreign accent syndrome resulting from acquired lesions in the left inferior frontal area, affecting the perisylvian region, and bilaterally in the pons. This is the first case of FAS reporting lesions within the pontine brainstem. The patient presented with distinct German accent and a waxing and waning of symptoms corresponding with level of arousal. This report provides support for conceptualizing FAS as a disorder involving all three functional cerebral units and the interplay of circuits involved in the regulation of arousal, emotion, and speech.
References
- Blumstein SE & Kurowski K. The foreign accent syndrome: A perspective. Journal of Neurolinguistics. 2006; 19.
- Marien P, Verhoeven J, Engelborghs S, Rooker S, Pickut BA, De Deyn PP. A role for the cerebellum in motor speech planning: evidence from foreign accent syndrome. Clin Neurol Neurosurg. 2006; 108: 518-522.
- Whiteside SP, Varley RA. A reconceptualisation of apraxia of speech: a synthesis of evidence. Cortex. 1998; 34: 221-231.
- Di Dio C, Schulz J, Gurd JM. Foreign accent syndrome: In the ear of the beholder? Aphasiology. 2006; 20.
- Bakker JI, Apeldoorn S, Metz LM. Foreign accent syndrome in a patient with multiple sclerosis. Can J Neurol Sci. 2004; 31: 271-272.
- Edwards RJ, Patel NK, Pople IK. Foreign accent following brain injury: syndrome or epiphenomenon? Eur Neurol. 2005; 53: 87-91.
- Marien P, Verhoeven J. Cerebellar involvement in motor speech planning: some further evidence from foreign accent syndrome. Folia Phonier Logo. 2007; 59: 210-217.
- Damasio AR, Anderson SW, Tranel, D. The frontal lobes. Heilman KM &Valenstein E (eds.), In: Clinical Neuropsychology. 2012; 417-465.
- Luria AR. A history of psychology in autobiography.1974.
- Marien P, van Dun K, Verhoeven J. Cerebellum and apraxia. Cerebellum. 2015; 14: 39-42.
- Harrison DW. Mixed Brain Syndromes. In Brain Asymmetry and Neural Systems. Springer International, Switzerland. 2015b; 263-265.
- Harrison DW. Positive and Negative Emotion. In Brain Asymmetry and Neural Systems. Springer International Publishing. 2015c; 389-413.
- Harrison DW. Brain Asymmetry and Neural Systems: Foundations in Clinical Neuroscience and Neuropsychology. Springer International, Switzerland. 2015a.