
Special Article - Tourette Syndrome
Austin J Neurol Disord Epilepsy. 2016; 3(2): 1020.
Botulinum Neurotoxin for the Treatment of Motor and Vocal Tics
Ganihong I, Rabin ML*, Kurlan R
Atlantic Neuroscience Institute, Overlook Medical Center, USA
*Corresponding author: Marcie L. Rabin, Atlantic Neuroscience Institute, Overlook Medical Center, 99 Beauvoir Ave, Summit, NJ, USA
Received: May 16, 2016; Accepted: July 02, 2016; Published: July 08, 2016
Abstract
Over the past twenty years, Botulinum neurotoxin (BoNT) has emerged as a potential new treatment for motor and vocal tics. This review examines the safety profile and efficacy of BoNT for these indications. A Medline literature search using the keywords botulinum toxin, Tourette’s syndrome, and tics was performed to identify relevant articles. Thirteen case reports, open-label studies, and randomized controlled trials were isolated and collected for review. Preliminary evidence from these studies suggests that BoNT can be a safe and effective treatment for tics. Though the mechanism through which BoNT suppresses tics is not clearly understood, it is notable that treatment with BoNT has been consistently reported to mitigate premonitory urges. Side effects are generally dependent upon on the injection site but are consistent with what has been observed with BoNT treatment for other hyperkinetic movement disorders. Much of the literature, however, consists of case reports and uncontrolled openlabel studies. Though initial results are promising, future studies are needed to better assess BoNT as a treatment for tics.
Keywords: Botulinum neurotoxin; Tourette’s syndrome; Vocal tics; Motor tics
Abbreviations
BoNT: Botulinum neurotoxin; TS: Tourette’s Syndrome; ona/ BoNT: OnabotulinumtoxinA; abo/BoNT: AbobotulinumtoxinA
Introduction
Tics are abnormal, repeated movements (motor tics) or sounds (vocal tics) that present with varying complexity. Premonitory urge is a commonly described feature of tic disorders and is a focal, uncomfortable sensation that can be alleviated by performing a tic. Tics are often classified as semi-voluntary, meaning that though patients can suppress the action for a time, discomfort increases until a tic is performed [1,2]. Motor tics can involve a single simple movement like eye-blinking or head jerking, or can involve a complicated series of movements that are done in the same order such as toe tapping followed by shoulder shrugging. Similarly, vocal tics, also known as phonic tics, can range from coughing or sniffing to vocalizations of entire words or phrases. Tourette’s Syndrome (TS) is a common neurodevelopmental disorder with childhood onset that is characterized by the presence of chronic motor and vocal tics [2]. A diagnosis of TS requires tic onset before 18 years of age, the presence of both motor and vocal tics, and symptom duration of at least 1 year. Other tic syndromes include chronic motor tic disorder and chronic vocal tic disorder. These diagnoses are made when an individual exhibits tics for more than 1 year and the tics are exclusively motor or vocal types, respectively. A recent meta-analysis of various tic disorders reported a prevalence rate for TS of 0.77% (95% confidence interval: 0.39-1.51) in the pediatric population and 0.05% (95% confidence interval: 0.05-0.08) in the adult population [3]. Tics may be disabling, and some studies have described an association between childhood TS and impaired quality of life, which may be related to feelings of social isolation and embarrassment [4,5]. Academic or work performance may also be affected in patients with tic disorders, as efforts to suppress tics hinder concentration and attention.
Current interventions for tic disorders include oral ticsuppressing medications and behavioral therapies. Antipsychotic drugs (haloperidol, pimozide, risperidone, aripiprazole) and a2- adrenoceptor agonists (clonidine, guanfacine) are commonly used. However, side effects of sedation, dizziness, and headache in a2-adrenoceptor agonists and sedation, depression, weight gain, glucose intolerance, and parkinsonism in antipsychotics may be intolerable. Additionally, pharmacotherapies may not provide adequate symptom control for some patients. Behavioral therapy, while attractive as a non-pharmacological intervention, may be timeintensive, questionably effective for more severe tics, and restricted by the limited availability of qualified psychologists [6,7]. Deep brain stimulation has more recently emerged as a possible treatment option, but studies have yielded mixed results, and a consensus has yet to be reached on the appropriate brain targets [8]. Thus, new treatments are needed for the management of tics.
Botulinum neurotoxin as a treatment for tics
Botulinum neurotoxins (BoNT) are a group of structurally related neurotoxic proteins produced by the bacterium Clostridium botulinum. Of the two commercially available serotypes, BoNT-A is more commonly used in clinical settings. BoNT-A is a zincdependent endopeptidase that cleaves SNAP-25, a protein involved in the fusion of synaptic vesicles with the presynaptic membrane [9]. BoNT treats most hyperkinetic movement disorders by impairing acetylcholine release at neuromuscular junctions, thereby temporarily chemodenervating overactive muscles and normalizing muscular activity [10]. BoNT has also been shown to be effective in blocking sensation, specifically pain. While the exact mechanism is not clearly understood, based on in vitro experiments, it is thought that BoNT may block the release of neuropeptides involved in pain from peripheral sensory nerves. It may also have a neuromodulatory effect on peripheral nocioceptive receptors and ion channels, indirectly affecting upstream pathways in parts of the brain responsible for pain perception [11]. In TS, it has been proposed that there is a loss of inhibition of motor programs/signals that are spontaneously generated in the brainstem [12]. BoNT may then be useful for tics either by chemodenervating muscles that would be activated by these aberrant signals, on by reducing the urge to tic through blocking sensory information, or by doing a combination of both. Beginning in the mid-1990s, several small studies have assessed the efficacy of BoNT for the treatment of motor and vocal tics. Presented here is a comprehensive review of the available literature on this topic.
Methods
We performed a Medline literature search for the keywords botulinum toxin in combination with Tourette’s syndrome and tics (Figure 1). All case reports, open-label studies, and randomized controlled trials were identified. This produced 13 articles for further examination. Many included only Tourette’s patients, but some also included patients with chronic motor tic disorder, tic duration of less than one year, and tic onset past 18 years of age. We then reviewed these publications for information relating to the efficacy and safety of BoNT in the treatment of motor and vocal tics.
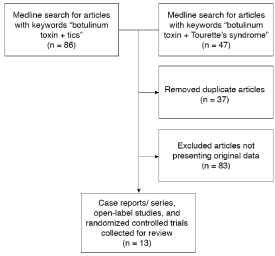
Figure 1: Flowchart representation of the Medline literature search.
Studies of motor tics
Three case reports, two uncontrolled open-label studies, and one double-blind, placebo-controlled study have investigated the use of BoNT for motor tics (Table 1). Case reports have documented BoNT as an effective treatment for ear wiggling tics [13]; dystonic neck tics [14]; and writhing tics with neck, shoulder, and trunk involvement [15]. Dosing in these reports was dependent on the targeted muscles, and subjects generally noticed improvement within one week of receiving the injections. In the cases of the dystonic neck and ear wiggling tics, BoNT treatment led to complete resolution of the tics at peak effect [13,14]. However, the subject with the dystonic neck tic reported continuing improvement over a two-month period and described sustained resolution of the tic after 12 months of follow-up. This is inconsistent with the general duration of effect of BoNT and suggests that spontaneous remission of the tic may have occurred. Regardless, BoNT was well tolerated and no side effects were reported in the three cases.
Reference
Design
Sample
Treatment
Adverse events
(# of Subjects; Duration)
Measured outcomes
Findings
Latency
Response duration
Srirompotong et al., 2007 [13]
Case report
One 35 y/o male with ear wiggling tic of 5 mo duration
40 U of BoNT-A (formulation unspecified) into the auricularis anterior, superior, and posterior.
No side effects reported
-
The subject reported almost complete cessation of his tic.
5 d
Unknown
Aguirreg-omozcorta et al., 2008 [14]
Case report
42 y/o male with refractory, dystonic motor tics involving the neck and shoulder
50 U of ona/BoNT into each sternocleidomastoid and 100 U into each splenius capitis. The patient underwent two treatments spaced 4 mo apart.
No side effects reported
-
Self-reported complete resolution of dystonic tics
Unk
At least 12 mo
Chen et al., 2010 [15]
Case report
11 y/o male with a writhing motor tic involving the neck, shoulder, and trunk. Tic movements were preventing the union of a clavicular fracture.
60 U of ona/BoNT into the pectoralis major, 40 U into the anterior deltoid, 50 U into the trapezius and 30 U into the levator scapulae
No side effects reported
-
BoNT was effective in alleviating his tic to allow fracture healing.
1 d
3-4 mo
Jankovic, 1994 [16]
Open-label study
10 male patients aged 13-53 with eye blinking or dystonic neck tics.
Ona/BoNT was injected into the muscles involved in the tics, with dosages depending upon the target muscle. Subjects received between 1-5 treatment sessions. Follow-up occurred between 2 and 4 months after the injections
Ptosis (2; 4 wks)
Neck weakness/pain (3; 2 wks)
The subject and one observer rated the peak efficacy on a scale of 0-4, with 0 describing no effect and 4 describing near complete resolution of the tics. Subjects also rated the effect on premonitory sensations on a scale between 0-3.
All 10 patients experienced at least some improvement in their tics. Out of 29 sessions between the 10 subjects, 27 resulted in moderate to marked improvement. BoNT provided moderate to complete relief of premonitory sensations for those subjects who had them. One subject experienced permanent resolution of his tics.
2-7 d
Mean was 14 ± 5.3 wks for those without permanent resolution
Rath et al., 2010 [17]
Open-label study
15 male and female patients aged 18-84 with chronic, intractable simple motor tics involving the eyes, face, neck, abdomen, or back.
Ona/BoNT injections were given every 3-4 months with total treatment duration ranging from 0.5 to 10 years
Development of new tic (1;unk)
Flu-like symptoms (1;unk)
Congestion (1;unk)
Muscle weakness (1;unk)
Reduced facial expression (1;unk)
Self-report of treatment efficacy scored on a 4 point scale (no effect, poor, moderate, good)
13 subjects reported good or moderate improvement. Of these 13, 3 subjects described permanent resolution of their tics, as defined by a tic-free period of at least 2 years. No effect was seen for 2 subjects.
2-7 d
Most patients reported duration as >12 wks
Marras et al., 2001 [18]
DB, RCT, Crossover design
18 male and female study completers aged 15-55 with simple motor tics involving the eyes, face, neck, or shoulder.
Ona/BoNT was injected into muscles associated with tics. Dosages were reported to be consistent with those used in dystonia. Follow-up at 2, 6, and 12 wks after treatment. Additional follow-up every 4 wks thereafter if the tics had not yet returned to baseline. Once at baseline, the subject was crossed over into the alternate phase.
Adverse events for the BoNT phase:
Subjective weakness (9;unk)
Weakness on examination (12; unk)
Neck discomfort (3;unk)
Blurred vision (1;unk)
Dysphagia (2;unk)
Restlessness (2;unk)
Increased urge to tic (1;unk)
Development of a new tic (2;unk).
Primary measure: Tics per minute as seen on videotape
Secondary measures: Shapiro Tourette Syndrome Severity Scale;
Self-reported urge to tic rated 0-4;
Self-reported intensity of premonitory sensation rated 0-4;
Global impression of change;
Frequency, intensity, and interference subsections of YGTSS;
Interference and suppression categories of the Unified Tic Rating Scale
A median 39% reduction of tics seen per minute was observed in the BoNT condition compared to a median 5.8% increase in the placebo phase (p = 0.0007). A mean 46% reduction in urge to tic was also seen with BoNT (p = 0.02). No other significant differences were seen in the secondary outcome measures, indicating that the subjects did not discern meaningful clinical benefit from treatment with BoNT.
Unk
Unk
Table 1: Summary of reports on BoNT for motor tics.
In 1994, Jankovic conducted the first open-label study of BoNT for simple motor tics [16]. He enrolled 10 subjects with either eyeblinking or dystonic neck tics and rated improvement on a 0 - 4 scale, with 0 signifying no effect and 4 representing a near complete or complete resolution of the tic. Each subject received between 1 to 5 treatment sessions, totaling 29 sessions between the 10 subjects. Average improvement at peak effect was 3.76, and 27 of the 29 sessions (93%) resulted in improvement rated as 3 or 4. Subjects began noticing improvement within 2-7 days after the injection, and of those without permanent resolution, the total duration of benefit was an average of 14 weeks. Notably, BoNT moderately or completely alleviated premonitory sensations in subjects who experienced them. Side effects were transient and included ptosis (2 subjects) and neck weakness/pain (3).
Rath et al. conducted a second uncontrolled open-label study for simple motor tics in 2010 [17]. Fifteen subjects with tics involving the eyes, face, neck, abdomen, or back were treated with BoNT every 3-4 months. Treatment lasted for 0.5 to 10 years. Improvement was rated on a four-point scale, and subjects were asked to evaluate BoNT as having no, poor, moderate, or good effect on their tics. Of the 15 treated subjects, 13 reported moderate or good improvement (87%) while 2 reported no effect (13%) after the first set of injections. Three subjects achieved tic-free periods of at least two years, suggesting that these patients may have had spontaneous remission of their tics. Latency to benefit was 2-7 days and improvement was sustained for most patients for 12 weeks or longer in those subjects who had temporary benefit. Adverse events included flu-like symptoms (1 subject), congestion (1), muscle weakness (1), reduced facial expression (1), and the development of a new tic (1). The duration of side effects was not reported. Treatment duration in this study is the longest of any published trial conducted for BoNT use in tics, with five subjects receiving treatment for at least four years. Four of these subjects reported equal efficacy and duration of response for their first and last injections, suggesting that long-term use of BoNT for this indication can provide consistent clinical benefit over time.
In 2001, Marras et al. conducted the only randomized, doubleblind, placebo-controlled study examining the use of BoNT for motor tics [18]. This trial had a crossover design with 18 study completers, all of whom had simple motor tics involving the eyes, face, neck, or shoulder. Relative change in tics per minute as measured during a videotaped session two weeks post-treatment was selected as the primary outcome measure. Measured tic frequency was compared to the pre-treatment assessment. Secondary outcome measures included self-report assessments for premonitory sensations, urge to tic, and global ratings of change. The median change in tic frequency in the BoNT phase was -0.39, indicating a 39% reduction in the treated tics, compared to a relative change of +0.058 in the placebo phase, indicating a 5.8% increase in tic frequency (p = 0.0007). Urge to tic
was also diminished with BoNT with a mean -0.46 point decrease observed with BoNT treatment compared to a +0.49 point increase in the placebo phase on a 0-4 point scale (p = 0.02). However, in contrast with previous reports, treatment with BoNT did not significantly alter premonitory sensations. The study failed to show improvement in other subjective measures of change as well, including the Shapiro Tourette Syndrome Severity Scale, the frequency, intensity, and interference subsections of the Yale Global Tic Severity Scale (YGTSS), and the interference and suppression items of the Unified Tic Rating Scale. Although videotape recordings demonstrated a decrease in tic frequency with BoNT, subjects did not perceive substantial clinical benefit from the treatment. Adverse events were relatively common in the BoNT phase and included subjective weakness (9 subjects), weakness on examination (12), neck discomfort (3), blurred vision (1), dysphagia (2), motor restlessness (2), increased urge to tic (1), and the development of a new tic (2). The authors suggested that the lack of improvement in secondary measures may have been due to insufficient study power as well as the inclusion of subjects with mild tics. At baseline, the median score of the YGTSS interference score was 1 out of 5, indicating that the targeted tics were not disabling or bothersome for many of the subjects enrolled, thus leading to a floor effect. The frequency of adverse events may have also modulated subjects’ impressions of overall response.
Studies of vocal tics
Fewer studies, which include one uncontrolled open-label trial, one case series, and three case reports, have investigated the effect of BoNT on vocal tics (Table 2). The case series and reports document a positive response of vocal tics to BoNT when tics were otherwise refractory to standard therapies [19-22]. All injections were given into the thyroarytenoid muscles. Two of these case reports examined the effect of BoNT on premonitory sensations and both found them to be alleviated by the injections [19,22]. Adverse events included breathy phonation [19-21], hoarseness [19,22], and dysphagia [19,21], all of which were transient. Duration of improvement was generally between 5 and 10 weeks.
Reference
Design
Sample
Treatment
Adverse events (# of Subjects; Duration)
Measured outcomes
Findings
Latency
Response duration
Vincent, 2008 [19]
Case series
Two patients aged 14 and 49 with refractory vocal tics
1.25-2.5 U of ona/BoNT was injected into the thyroarytenoid muscles. The 49-year-old underwent 6 treatment sessions, and the 14-year-old completed 3.
Breathy phonation (2; 7-30 d)
Hoarseness (2; 7-10 d)
Mild dysphagia (1; 2-3 d)
Used 0-4 point scale to measure efficacy of ona/BoNT, change in premonitory urge, and change in disability caused by tics.
Both patients experienced good efficacy from the BoNT injections after a titration period and reported improvement in all measured outcomes.
Unk
10 wks (mean)
Trimble et al., 1998 [20]
Case report
One 34-year-old male patient with various motor tics, coprolalia, and echolalia
3.75 U of abo/BoNT was administered into each thyroarytenoid muscle.
-
Breathy phonation and aspiration with liquids lasting several days
Improvement was seen in volume and control over the patient's voice, allowing previously loud vocal tics to blend in with normal speech.
Unk
Unk
Salloway et al., 1996 [21]
Case report
One 28-year-old male with screaming tics, coprolalia, and motor tics.
1.25-3.75 U of ona/BoNT was injected into each thyroarytenoid muscle every three mo for over a year
Transient
breathy phonation and dysphagia
Unified Spasmodic Dysphonia Rating Scale (USDRS)
Yale Global Tic Severity Scale (YGTSS)
Two point reduction in tic volume on USDRS; reduction in YGTSS intensity, interference, and impairment scores from marked-severe to moderate; reduction in YGTSS frequency score from always to frequent; self-reported 50% decrease in tic volume
Unk
Approx. 9 wks
Scott et al., 1996 [22]
Case report
One 13-year-old male patient with severe coprolalia, other simple vocal tics, and multiple motor tics
30 U of ona/BoNT was administered to the left vocal cord.
Hoarseness lasting several wks
-
The patient reported a 75% decrease in coprolalia and a decrease in premonitory urge.
2-3 d
Approx. 5 wks
Porta et al., 2003 [23]
Open-label study
30 subjects aged 10-65 with vocal tics
2.5 U of ona/BoNT was injected into right and left vocal cords. Subjects received an average of 1.9 ± 1.0 treatment sessions. Follow-up occurred 15 days post-treatment and then an additional 4 times in 12 mo.
Hypophonia (24; 10 ± 3 d)
Tic complexity (simple, complex, both);
Hopkins vocal tic scale;
Global impression of change;
Evaluation for presence of premonitory urge
93% of subjects experienced some improvement from BoNT. 50% experienced complete cessation of their tic. 63% of subjects with premonitory urges reported complete resolution of the urge.
Tic frequency was decreased for some. Subjects also reported a reduction in their tics' interference in their social and work lives.
5.8 d (mean)
14.6 wks (mean)
Table 2: Summary of reports on BoNT for vocal tics.
The only published study of BoNT for the treatment of vocal tics exclusively is an uncontrolled, open-label trial conducted by Porta et al. in 2003 [23]. Improvement in this study was measured by a change in tic complexity. At baseline, subjects were characterized as having simple, complex, or both simple and complex vocal tics, and transition to a simpler tic or to no tic post-treatment was considered to be improvement. Injections of 2.5 U of BoNT into the left and right vocal cords produced improvement in vocal tics for 28 out of 30 (93%) subjects [23]. The effects were robust, with 15 subjects (50%) experiencing complete resolution of their vocal tics. Tic frequency, as measured by the Hopkins Vocal Tic Scale, was also improved. BoNT was additionally successful in fully suppressing premonitory sensations for 10 out of the 16 subjects (63%) who reported such symptoms. Latency to onset of improvement was an average of 5.8 days, and the treatment effect lasted an average of 14.6 weeks (range: 2.9 - 42.9 weeks). Hypophonia was reported by 24 (80%) subjects and resolved within 10 ± 3 days. Notably, subjects reported that BoNT treatment mitigated the interference of their vocal tics in their work, school, and social lives.
Studies of motor and vocal tics
Two additional uncontrolled open-label trials recruited a cohort of subjects with motor and/or vocal tics, but the effects of BoNT on tics were not independently examined according to these two types of tics (Table 3). Kwak et al. enrolled 35 subjects, some of whom had multiple tics that were targeted with BoNT, and allowed for BoNT dose titration [24]. Motor tics comprised the majority of tics assessed in this study, with only four subjects receiving BoNT injections into the vocal cords. Twenty-nine of the 35 subjects (83%) experienced at least some benefit from BoNT treatment. Using a 0-4 point scale, 23 of the 29 rated improvement as a 3 or 4, indicating a moderate to marked reduction in tic severity and functional impairment. Premonitory sensations were also substantially alleviated, with 21 of 25 subjects (84%) reporting relief from these symptoms. The mean latency to benefit was 3.8 days, with total duration of improvement averaging 14.4 weeks. Notably, 5 subjects saw sustained resolution of their tics even after one year, which may be reflective of spontaneous remission rather than BoNT-related improvement. Although it was not determined quantitatively how effective the injections were for vocal tics, the authors remarked that subjects with vocal tics responded particularly well to treatment. Adverse events for the full study cohort included generalized weakness (1 subject), fatigue (1), ptosis (2), nausea (1), neck weakness (4), dysphagia (2), and hypophonia (1).
Reference
Design
Sample
Treatment
Adverse events
(# of Subject; Duration)
Measured outcomes
Findings
Latency
Response duration
Kwak et al., 2000 [24]
Open-label study
35 male and female patients aged 8-69 with motor tics or vocal tics.
Ona/BoNT was given to the muscles involved in the tic with dosages depending on the target muscle. A total of 115 treatment sessions were captured. Follow-up occurred within 1 year of treatment.
Neck weakness (4; 23 d)
Ptosis (2; 28 d)
Generalized weakness (1; 1 wk)
Dysphagia (2; 17.5 d)
Fatigue (1; 2 wks)
Nausea/ vomiting (1; 1 d)
Efficacy of injections at peak effect was rated on a 0-4 point scale, with 0 being no effect and 4 describing marked effect. Percentage improvement of premonitory sensation was also assessed.
29 patients saw improvement with BoNT, and 23 saw improvement rated 3 or 4. Five patients saw sustained, complete resolution of their tics at 1 year follow up. 21/25 patients with premonitory sensations reported improvement. Out of 115 total sessions, 78 resulted in 3+ improvement. The authors note that vocal tics and tics with eyelid involvement appeared most responsive to BoNT.
3.8 ± 2.9 d
14.4 ± 10.3 wks
Awaad, 1999[25]
Open-label study
186 male and female patients aged 6-18 with motor or vocal tics.
Ona/BoNT was injected into the muscles associated with the tic every 6-9 mo. However, 23 subjects received injections every 13-19 mo due to long duration of response.
Muscle soreness (5; unk)
Neck weakness (4; unk)
Ptosis (3; unk)
YGTSS;
Tic Symptoms Self-Report;
Conners Parent Rating Scale; Number of tics seen on a 2.5-minute video segment
4 subjects with vocal tics experienced 30% improvement with BoNT. 35 subjects saw complete resolution of their motor tics. No further information is provided, though the author notes that motor tics were more responsive to BoNT than were vocal tics.
Unk
Unk
Table 3: Summary of reports on BoNT for motor and/or vocal tics.
In a second uncontrolled open-label study, Awaad recruited 450 subjects with motor and/or vocal tics [25]. The subjects were divided into two active treatment groups: 264 were dispensed oral baclofen and 186 received BoNT. In the BoNT group, vocal tics were targeted with administrations of 5 - 10 U of BoNT into the cricoarytenoid/ thyroarytenoid muscles. With this regimen, 4 subjects injected for vocal tics achieved at least 30% improvement. He also reported that 35 subjects achieved complete resolution of their motor tics with BoNT. He did not provide results for the remaining subjects, nor did he report results from the rating scales administered to any of the subjects. Because of missing quantitative data, it is difficult to evaluate the magnitude of effects of BoNT in this study. Adverse events from the full BoNT cohort included soreness (5 subjects), neck weakness (4), and ptosis (3).
Discussion
Based on the available evidence, in 2008 the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology concluded that BoNT is possibly effective for the treatment of motor tics, and that there is insufficient evidence to determine its efficacy for vocal tics [26]. There was no update in the 2016 Guidelines [27]. The safety and tolerability of BoNT for tics reported is largely consistent with what has been reported in BoNT therapy for other movement disorder indications [10]. Side effects are generally focal and dependent upon the injection site. For example, neck weakness is more likely with injections into neck muscles and ptosis is more common with injections of the orbicularis oculi. Two studies reported the development of a new tic as a side effect of BoNT, which could be a risk unique to this indication [17,18]. Unlike other movement disorders, the natural history of TS is to wax and wane in severity, and this may involve spontaneous remission of a tic or the development of a new one. It is possible that this side effect was coincidental and subjects would have developed new tics even without BoNT. However, one study reported that the cessation of BoNT therapy was associated with the return of the subject’s previously treated tic and the elimination of the newly developed movement [17]. Larger placebo-controlled studies are needed to further characterize this phenomenon.
While evidence is more limited supporting BoNT treatment for vocal tics, patients with bothersome vocal tics may still benefit. However, there is a need for further investigation, as no randomized controlled trials have yet been conducted for this particular indication. Importantly, the previously published studies have demonstrated the general safety and tolerability of BoNT when used to treat vocal tics, as side effects were reported to be mild, transient, and similar to what has been reported for injections for laryngeal dysphonia and laryngeal tremor [10,28,29]. Hypophonia, breathy phonation, hoarseness, and dysphagia are most common. A latency of several days is expected before improvement is first observed, and duration of response is largely consistent with what has been reported for BoNT treatment of other movement disorders [10].
The impact of BoNT on premonitory sensations is also noteworthy. Several studies of motor and vocal tics found premonitory sensations to be mitigated by BoNT in a majority of subjects that experienced such symptoms [16,23,24]. A study of 50 subjects with TS found that 71% who had premonitory sensations believed that their motor tics would resolve if the sensations were eliminated [1]. It is then possible that the alleviation of premonitory symptoms underlies at least some of the clinical improvement seen with BoNT. However, patients who do not have premonitory sensations may still report benefit from this treatment, and the one available randomized controlled trial did not find an effect from BoNT on premonitory urge [18]. Thus, the mechanism by which BoNT alleviates tics remains to be elucidated.
It is also important to comment upon the safety of BoNT in the pediatric population, as this group suffers disproportionately from tic disorders. Several of the studies discussed here have included subjects less than 18 years of age. The only study that specifically links adverse events to their corresponding subjects is Jankovic 1994 [16]. Six of his 10 subjects were under 18 years of age, and three experienced side effects from treatment with BoNT (50%) of his four adult subjects, two suffered adverse events (50%). Other studies have also recruited subjects as young as six [25], eight [24], ten [23], and fifteen [18] with no reports of serious adverse events in their cohorts. For these studies, it is unknown how many pediatric subjects were recruited and what side effects they in particular experienced. However, adverse effects reported across these studies tended to be mild, suggesting that treatment with BoNT may be safe in this population. It should also be noted that BoNT has already been used in the treatment of other disorders in pediatric groups. A retrospective study of 45 children and adolescents showed that doses of onabotulinumtoxinA between 75 - 200U were well tolerated in treating chronic migraine, with only 8 of 131 (6.1%) treatment sessions resulting in side effects [30]. Doses of 4U/kg body weight of onabotulinumtoxinA (200U maximum) have also been used to treat equines foot deformity in children with cerebral palsy, and no serious adverse events related to BoNT were reported in an open-label study of over 200 subjects [31]. Based on the available literature, the 2009 European consensus on the use of botulinum toxin in children with cerebral palsy recommended a maximum total dose of up to 20U/kg body weight [32].
Conclusion
Overall, BoNT shows promise as a potential treatment for tics that are refractory to standard pharmacological therapies. Between the 13 studies presented here, a total of 302 patients have been trialed on BoNT for motor/vocal tics. The available literature indicates that BoNT may be effective in mitigating tic frequency and severity, diminishing premonitory sensations, and reducing functional impairment.
If shown to be effective, BoNT has advantages over conventional treatments. BoNT is injected directly into target muscles, and side effects are usually mild and focal. Patients who do not tolerate oral medications may then tolerate BoNT. Treatment with BoNT also requires a lesser time commitment from the patient compared to behavioral therapy. Thus, BoNT may improve treatment options for many patients with tic disorders who do not respond to standard therapies, or who do not tolerate them due to side effects.
The main limitation of this review is the lack of available literature, especially recent literature. Unfortunately, carefully done studies of this treatment approach remain sparse with only one doubleblind, placebo-controlled trial having been published that suffered several methodological shortcomings and focused only on motor tics. No controlled trials have been reported for vocal tics. Further, few studies have utilized validated rating scales, instead using selfcreated measures, which complicates inter-study comparison. Additionally, only two types of BoNTs have been used in these reports: onabotulinumtoxinA (Botox®) and abobotulinumtoxinA (Dysport®). Two other commercially available formulations, incobotulinumtoxinA (Xeomin®) and rimabotulinumtoxinB (Myobloc®), have not yet been studied in this context. Ultimately, future studies are needed to better substantiate the efficacy of BoNT in the treatment of tics, and more information is needed to better elucidate the mechanisms by which BoNT might alleviate tics.
References
- Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette's syndrome. Mov Disord. 2003; 18: 1530-1533.
- Kurlan R. Clinical practice. Tourette's Syndrome. N Engl J Med. 2010; 363: 2332-2338.
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012; 47: 77-90.
- Elstner K, Selai CE, Trimble MR, Robertson MM. Quality of Life (QOL) of patients with Gilles de la Tourette's syndrome. Acta Psychiatr Scand. 2001; 103: 52-59.
- Cutler D, Murphy T, Gilmour J, Heyman I. The quality of life of young people with Tourette syndrome. Child Care Health Dev. 2009; 35: 496-504.
- Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011; 26: 1149-1156.
- Müller-Vahl KR, Roessner V. European Society for the Study of Tourette Syndrome . Treatment of tics in patients with Tourette syndrome: recommendations according to the European Society for the Study of Tourette Syndrome. Mov Disord. 2011; 26: 2447.
- Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. 2015; 30: 448-471.
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin a selectively cleaves the synaptic protein SNAP-25. Nature. 1993; 365: 160-163.
- Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004; 75: 951-957.
- Kumar R, Dhaliwal HP, Kukreja RV, Singh BR. The Botulinum Toxin as a Therapeutic Agent: Molecular Structure and Mechanism of Action in Motor and Sensory Systems. Semin Neurol. 2016; 36: 10-19.
- Mink JW. Basal ganglia dysfunction in Tourette's syndrome: a new hypothesis. Pediatr Neurol. 2001; 25: 190-198.
- Srirompotong S, Saeseow P, Kharmwan S, Srirompotong S. Ear wiggling tics: treatment with botulinum toxin injection. Eur Arch Otorhinolaryngol. 2007; 264: 385-387.
- Aguirregomozcorta M, Pagonabarraga J, Diaz-Manera J, Pascual-Sedano B, Gironell A, Kulisevsky J. Efficacy of botulinum toxin in severe Tourette syndrome with dystonic tics involving the neck. Parkinsonism Relat Disord. 2008; 14: 443-445.
- Chen Y, Thalayasingam P. Botulinum toxin to control an incapacitating tic in a child with a clavicular fracture. Anaesth Intensive Care. 2010; 38: 1106-1108.
- Jankovic J. Botulinum toxin in the treatment of dystonic tics. Mov Disord. 1994; 9: 347-349.
- Rath JJ, Tavy DL, Wertenbroek AA, van Woerkom TC, de Bruijn SF. Botulinum toxin type A in simple motor tics: short-term and long-term treatment-effects. Parkinsonism Relat Disord. 2010; 16: 478-481.
- Marras C, Andrews D, Sime E, Lang AE. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001; 56: 605-610.
- Vincent DA. Botulinum toxin in the management of laryngeal tics. J Voice. 2008; 22: 251-256.
- Trimble MR, Whurr R, Brookes G, Robertson MM. Vocal tics in Gilles de la Tourette syndrome treated with botulinum toxin injections. Mov Disord. 1998; 13: 617-619.
- Salloway S, Stewart CF, Israeli L, Morales X, Rasmussen S, Blitzer A, et al. Botulinum toxin for refractory vocal tics. Mov Disord. 1996; 11: 746-748.
- Scott BL, Jankovic J, Donovan DT. Botulinum toxin injection into vocal cord in the treatment of malignant coprolalia associated with Tourette's syndrome. Mov Disord. 1996; 11: 431-433.
- Porta M, Maggioni G, Ottaviani F, Schindler A. Treatment of phonic tics in patients with Tourette's syndrome using botulinum toxin type A. Neurol Sci. 2004; 24: 420-423.
- Kwak CH, Hanna PA, Jankovic J. Botulinum toxin in the treatment of tics. Arch Neurol. 2000; 57: 1190-1193.
- Awaad Y. Tics in Tourette syndrome: new treatment options. J Child Neurol. 1999; 14: 316-319.
- Simpson DM, Blitzer A, Brashear A, Comella C, Dubinsky R, Hallett M, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008; 70: 1699-1706.
- Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016; 86:1818-1826.
- Adler CH, Bansberg SF, Hentz JG, Ramig LO, Buder EH, Witt K, et al. Botulinum toxin type A for treating voice tremor. Arch Neurol. 2004; 61: 1416-1420.
- Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 2015; 125: 1751-1757.
- Kabbouche M, O'Brien H, Hershey AD. OnabotulinumtoxinA in pediatric chronic daily headache. Curr Neurol Neurosci Rep. 2012; 12: 114-117.
- Koman LA, Brashear A, Rosenfeld S, Chambers H, Russman B, Rang M, et al. Botulinum toxin type a neuromuscular blockade in the treatment of equinus foot deformity in cerebral palsy: a multicenter, open-label clinical trial. Pediatrics. 2001; 108: 1062-1071.
- Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A, et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol. 2010; 14: 45-66.