
Research Article
Austin Neurol & Neurosci. 2016; 1(1): 1003.
Outcomes of Decompressive Hemicraniectomy for Spontaneous Intracerebral Hemorrhage: A Case-Control Study
Shah SO1,2,3,4*, Rincon F1,2,3,4 and Bar B1,2,3,4
¹Departments of Neurology, Thomas Jefferson University, Philadelphia, PA USA
²Neurosurgery, Thomas Jefferson University, Philadelphia, PA USA
³Divisions of Critical Care and Neurotrauma, Thomas Jefferson University, Philadelphia, PA USA
4Cerebrovascular Diseases, Thomas Jefferson University, Philadelphia, PA USA
*Corresponding author: Syed Omar Shah, Department of Neurological Surgery, Thomas Jefferson University and Jefferson College of Medicine, Philadelphia
Received: October 22, 2015; Accepted: February 22, 2016; Published: February 24, 2016
Abstract
Introduction: Intracerebral hemorrhage (ICH) remains a devastating type of stroke. Besides blood pressure control, treatment options including surgery remain limited. Decompressive hemicraniectomy (DHC) in patients with spontaneous ICH may be considered as salvage therapy. The purpose of this study is to evaluate the role of DHC for the management of primary ICH associated with malignant cerebral edema and its effect on case-fatality and long-term functional outcome.
Methods: A matched case control study was conducted at Thomas Jefferson University. Patient records were reviewed for age, sex; Glasgow Coma Scale (GCS) and ICH score on admission, on discharge and at follow-up. For those patients that underwent DHC, the time to surgery was documented. Computed tomography scans were then reviewed at the time of admission, allowing calculation of ICH scores. Functional outcome was assessed over the phone using the Simplified Modified Rankin Scale Questionnaire (smRSq). We matched (1:1) our cases to controls (ICH without DHC) by age and ICH score. The primary outcome of interest was survival over time. Secondary outcomes were discharge GCS and long-term functional outcome measured by the Modified Rankin Scale (mRS).
Results: There were 17 patients with DHC who were matched (1:1). The mean time to DHC was 61.3 ± 101 hours. Median mRS at follow-up was similar in DHC group vs. controls (4, IQR 4-6 vs. 5, IQR 3-6, p = 0.7).In multivariable analysis, predictors of death overtime were ICH score > 2, and female gender. Predictors of survival was DHC but only in patients with ICH score > 2 (HR 0.83, 95% CI 0.7-0.9, p = 0.04 for interaction).
Conclusion: This case-control study suggests that patients with ICH associated with significant cerebral edema and an ICH score greater than 2 may potentially benefit from DHC. Further research in a larger patient population conducted prospectively is warranted to assess the potential benefit of DHC in ICH.
Keywords: Decompressive hemicraniectomy; Intracerebral hemorrhage; Stroke; Refractory intracranial pressure
Introduction
Morbidity and mortality from Intracerebral Hemorrhage (ICH) are important public health problems [1,2]. Hypertensive ICH accounts for 10%–20% of strokes in the United States. About 40-80% of ICH patients die within the first 30 days and half of all deaths occur within the first 48 hours [3]. Survivors usually require long-term health care [2]. To date, there is no effective therapy for the treatment of ICH and most importantly, mortality remains unchanged [4]. The 30-day mortality rate for patients with ICH volumes greater than 50–60 cc range from 81%-91%, and poor functional outcome rates of 96%–97% have been reported for those with ICH volumes greater than 40–45 cc [5-8].
Current guidelines developed from the American Heart Association and American Stroke Association (AHA/ASA) recommend initial therapy including: relief of intracranial pressure (External Ventricular Drainage (EVD)), blood pressure control, correction of coagulopathies, seizures control, fever prevention, glycemic control, and DVT prophylaxis [8]. Trials have been focused on hematoma evacuation alone, such as The International Surgical Trial in Intracerebral Hemorrhage (STICH) [9]. Hematoma evacuation in cerebellar hemorrhage with brainstem compression and/or hydrocephalus is strongly recommended [8]. Furthermore, lobar clots > 3cc and within 1 cm of the surface should also be considered for evacuation [8]. STICH-II study was recently published and its goal was to determine whether hematoma evacuation within 12 hours would improve outcome compared with medical management [10]. Despite showing non-significant results on the primary endpoint, early surgery did not increase the rate of death or disability at 6 months and suggested a small survival advantage for patients with ICH who do not have intraventricular hemorrhage. Preliminary results from the Minimally Invasive Surgery plus rt-TPA for Intracerebral Hemorrhage Evacuation (MISTIE-II) demonstrated efficacy in the treatment of ICH by using stereotactic evacuation plus intracavitary administration of rt-TPA [11]. Although the guidelines address surgical management, they do not discuss the utility of Decompressive Hemicraniectomy (DHC) for management of malignant cerebral edema secondary to ICH.
DHC is a surgical option to reduce ICP, increase cerebral compliance and increase cerebral blood perfusion when medical management becomes inadequate. Unfortunately, there have only been a few observational studies evaluating DHC for ICH, and these studies are divided regarding whether to also perform a hematoma evacuation along side with DHC [12-20].
The purpose of this study is to evaluate the role of DHC for the management of primary ICH associated with malignant cerebral edema and its effect on case-fatality and long-term functional outcome. We hypothesized that after adjustments for known predictors of poor outcome, DHC after ICH would be associated with improved in-hospital case-fatality and long-term functional outcome.
Methods
We conducted a matched case control study. This study was conducted with the approval of the IRB of Thomas Jefferson University Hospital. Admissions at our institution with a primary diagnosis of ICH (ICD-9-CM, 431) with and without DHC (ICD-9-CM, 01.2) were identified by querying of the hospital discharge database [3]. From January 2011 to November 2014, 17 consecutive patients with ICH underwent a DHC in our institution. Patients included were over the age of 18 with primary spontaneous ICH. Exclusion criteria included patients who had ICH secondary to infratentorial origin, AVM, secondary to bleeding into tumor or stroke, TBI, and coagulopathy. Patients who had a pre-morbid modified Rankin scale mRS greater than 2 were also excluded. A board certified vascular neurologist or neurointensivist internally validated the inclusion and exclusion criteria.
Patient records were reviewed for age, sex; Glasgow Coma Scale (GCS) and ICH score on admission, on discharge and at follow-up. For those patients that underwent DHC, the time to surgery was documented. The laterality of the hematoma along with location, hematoma volume, midline shift, and the presence of Intraventricular Hemorrhage (IVH) were recorded. Admission hematoma volumes were measured using a validated semi-automated method. Volumes were estimated on 5-mm-thin slices of admission and follow-up CT scans using Osirix 64 Bit 5.9 software (https://www.osirix-viewer. com). The software was used to generate a polygonal region of interest from a range of Hounsfield units of 40-100. Regions of interest on each axial cut were then manually checked and adjusted to include any missing hemorrhage. Subsequently, the software computed the volume of the polyhedron defined by the regions of interests in cubic centimeters using a Power Crust reconstruction filter.
Patients were also evaluated using the ICH score. The ICH score is a validated measure of ICH severity used in research and clinical trials in ICH [21,22]. The components of the ICH score are: age, GCS, hematoma volume (ml), location (infratentorial vs. supratentorial), and presence of Intraventricular Hemorrhage (IVH) [21].
We matched (1:1) our cases to controls (ICH without DHC) by age and ICH score. All patients received maximal medical therapy which included hyperosmolar treatment (Mannitol, 3% Saline), mechanical ventilation, glycemic control, blood pressure management and analgesic sedation as appropriately needed. Intracranial pressure (ICP) monitoring was not undertaken routinely.
The primary outcome of interest was survival over time. Secondary outcomes were discharge GCS and long-term functional outcome measured by the Modified Rankin Scale (mRS). Poor outcome was defined as mRS 5-6. The mRS is a validated ordinal scale of neurological function ranging from 0 or no deficit to 6 or death [23]. mRS outcomes were collected for ICH survivors using a structured and validated telephone questionnaire [24].
Surgical Approach
The decision to pursue DHC was based on the collective judgment of the treating neurointensivists, attending neurosurgeon, and patient/family preferences. The laterality of the hemorrhage did not impact decisions to proceed with a DHC. All patients received either a unilateral frontal or fronto-parietal-occipital craniectomy based on the localization of the hemorrhage. The minimum diameter opening for the bone in all cases was at least 12 cm. The durotomies were performed in a cruciate fashion. The decision to perform clot evacuation along with the DHC was left to the neurosurgeon. Overall 24% of patients received clot evacuation. Duraplasty was achieved with either bovine pericardium or DuraMatrix (Stryker, Kalamazoo, MI). All patients had a JP drain inserted and remained in for 1-2 days postoperatively.
Statistical Analyses
Continuous variables were assessed for normality using the Kolmogorov-Smirnov test and reported using accepted standards for parametric and nonparametric data as means and Standard Deviations (SD) or medians and Interquartile Ranges (IQRs). Categorical variables were reported as count and proportions in each group. Bivariate comparisons were made using the t-test or Mann- U-Whitney test for continuous variables and Χ² or Fisher’s exact test for categorical variables. Missing data from patient’s lost-to follow up (two patients in the DHC group and one in the control group) were treated by carrying forward the last known outcome of interest. To account for differences in time to follow-up, a multivariable analysis using parametric survival models were fitted with time to event as the dependent variable and censoring for the outcome of interest (death). The main co-variate of interest (case (DHC) status) and additional factors associated with mortality after ICH were included in the models. Parsimonious models were found by systematically removing the least significant factor and recalculating the model. Multiple survival distributions were compared and the AIC or likelihood ratio test allowed us to assess relative model goodness of fit. To this end, the Weibull distribution best fitted the data. Finally, we calculated the Hazard Ratios (HR) and their 95% CI and studied potential interactions between covariates of interest. Statistical analyses were conducted using JMP software version 11.0 (SAS, Cary NC). Our reporting of observational data conforms to the Strengthening the Reporting of Observational Studies in Epidemiology STROBE guidelines [25].
Results
Demographics, admission severity scores, and radiological characteristics are summarized in (Table 1). There were 17 patients with DHC who were matched 1:1 by age and ICH score to historical controls. The mean time to DHC was 61.3 ± 101 hours. CT findings demonstrated a statistically significant difference in median admission midline shift in patients undergoing DHC (8mm, Interquartile Range (IQR) (4-12mm) vs. 3mm, IQR (2-8mm), p = 0.05). Other radiological findings were similar between the two cohorts (Table 1). There were no patients who had a hemorrhage of infratentorial origin.
All n=34
Case (DHC) n=17
Control n=17
P value
Age, X (SD)
51 (16)
52 (16)
52 (16)
0.9
Gender (Female)
14 (41)
7 (50)
7 (50)
0.9
Race (White)
17 (50)
6 (38)
11 (65)
0.3
Comorbidities
Hypertension
27 (77)
13 (38)
14 (40)
0.5
Dyslipidemia
7 (21)
3 (8)
4 (12)
0.6
Diabetes Mellitus
6 (18)
4 (12)
2 (6)
0.4
Atrial Fibrillation
3 (9)
0 (0)
3 (8)
0.2
COPD
3 (9)
1 (3)
2 (6)
0.9
CHF
1 (3)
0 (0)
1 (3)
0.9
Admission scores
GCS Admission, Med (IQR)
10 (7-13)
7 (6-13)
10 (7-14)
0.4
ICH Score, Med (IQR)
2 (1-3)
2 (1-3)
2 (1-3)
0.9
CT Characteristics
Hematoma size, ml Med (IQR)
50 (34-101)
52 (44-107)
49 (25-84)
0.2
IVH, N (%)
21 (62)
8 (47)
13 (76)
0.07
Midline shift, mm Med (IQR)
6 (2-9)
8 (4-12)
3 (2-8)
*0.05
Basal Ganglia Origin, N (%)
21 (62)
11 (74)
10 (60)
0.6
Right hemisphere, N (%)
20 (58)
8 (47)
12 (71)
0.2
Time to follow up, days X (SD)
967 (374)
820 (381)
1114 (313)
*0.02
Table 1: Demographic and CT characteristics of Decompressive Hemicraniectomy (DHC) group and medical management group (control).
In bivariate analysis, overall mortality was lower in DHC compared to control group (29 % (5/17) vs. 47% (8/17), p = 0.2), although not reaching statistical significance. Although GCS upon discharge was higher in the control group, this did not affect longterm outcome (Table 2). Figure 1 shows the distributions of the raw scores on the mRS at follow-up between the two cohorts. Median mRS at follow-up was similar in DHC group vs. controls (4, IQR 4-6 vs. 5, IQR 3-6, p = 0.7).
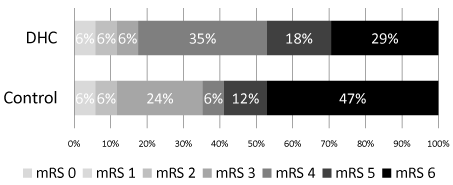
Figure 1: Outcomes after Decompressive Hemicraniectomy (DHC) compared
to medical management only (control). mRS – modified Rankin Scale.
Outcomes
All
DHC
Control
P Value
GCS on discharge, Med(IQR)
14 (12-15)
13 (11-14)
15 (14-15)
*0.04
mRS on discharge, Med(IQR)
5 (4-5)
5 (4-5)
5 (4-6)
0.7
mRS on follow-up, Med(IQR)
4 (3-6)
4 (4-6)
5 (3-6)
0.7
mRS 5-6 (%)
15 (48)
6 (40)
9 (56)
0.4
Mortality (%)
13 (38)
5 (29)
8 (47)
0.2
Table 2: Outcomes of Decompressive Hemicraniectomy (DHC) group and medical management group (control).
There was a difference between time to follow-up between the two cohorts (Table 1), secondary to the controls being historical and therefore had more time to event, which was death. In the multivariable analysis, predictors of death overtime were ICH score > 2, and female gender. Predictor of survival was DHC, but only in patients with ICH score > 2 (HR 0.83, 95% CI 0.7-0.9, p = 0.04 for interaction) (Table 3).
Mortality at Follow Up
B
SE
HR
95% CI
P Value
DHC Group
-0.218
0.11
0.80
0.648-0.998
§0.03
ICH Score > 2
0.199
0.09
1.22
1.023-1.456
§0.04
Gender, female
0.1
0.1
1.11
0.908-1.344
0.46
Midline shift on admission > 6mm
-0.01
0.1
0.99
0.814-1.204
0.93
DHC patients with ICH Score > 2*
-0.191
0.09
0.83
0.693-0.986
§0.04
ICH – Intracerebral Hemorrhage, B – Regression Coefficient, SE – Standard Error, HR – Hazard Ratio, CI – Confidence Interval, GCS – Glasgow Coma Scale, mRS – Modified Rankin Scale, IQR – Interquartile Range, *interaction-term, §significant at a level 0.05.
Table 3: Multivariate Parametric Survival Analysis for patients with Decompressive Hemicraniectomy (DHC), interaction term.
Discussion
Our multivariable analysis shows that patients who received a DHC with an ICH score of > 2 had a 17% decrease in the odds of death over time (p = 0.04 for interaction, (Table 3)). Thus, patients with higher ICH scores and who were more likely to have poor outcomes appeared to benefit the most from DHC in the long-term.
DHC has been studied in severe Traumatic Brain Injury (TBI), high-grade aneurysmal Subarachnoid Hemorrhage (SAH), and malignant cerebral infarction [26-30]. However, the neuroprotective effect of DHC has only been established in clinical trials of patients with hemispheric ischemic stroke [26]. There was a clear benefit for mRS > 4 cutoff (medical 76%, surgical 25%, P < 0.0001) and mortality at 1 year (medical 71%, surgical 22%, P < 0.0001). This analysis demonstrated an absolute risk reduction of 49% with a number needed to treat of only 2. Additionally, with the pooled data, there was a statistically significant difference between the groups for a mRS > 3 at 12 months (medical 79%, surgical 57%, P = 0.014).
There are currently no large randomized controlled trials regarding the use of DHC in ICH. Trials in specific populations of ICH patients have been focused on hematoma evacuation alone, such as The International Surgical Trial in Intracerebral Hemorrhage (STICH) [9]. This landmark trial demonstrated that emergent surgical hematoma evacuation of superficial lobar hemorrhages within 72 hours of onset failed to improve outcome compared with standard medical management [9]. The results of STICH-II demonstrated that early surgery did not improve the rate of death or disability at 6 months but suggested a small survival advantage for patients with ICH who did not have IVH or hydrocephalus [10]. There has also been some preliminary data from the MISTIE-II investigators suggesting that the use of recombinant Tissue-Type Plasminogen Activator (tPA) along with Minimally Invasive Surgery (MIS) via a catheterbased approach may be associated with cost-benefits and improved outcomes at 1-year [31]. Along with demonstrating the safety of MIS + catheter based tPA, the MISTIE-II study showed that greater clot reduction was associated with lower peri-hematoma edema [11]. The phase-III of this trial is currently underway. Of note, patients in these clinical trials differed in disease severity compared to patients in our case control study. For example, in STICH-II, 50% or more had GCS > 13 and initial hematoma volume was 38 mL (IQR, 24–62) [9]; in MISTIE-II, the mean admission GCS was 11 ± 3 and the hematoma volume was 35 mL ± 18 [31]. This suggests that in certain ICH subpopulations, that would not otherwise meet criteria for MIS, DHC may be a surgical option to improve survival.
In our cohort, DHC was performed as a life-saving procedure to manage the effects of refractory intracranial hypertension and cerebral edema seen on imaging and clinical exam. Intracranial pressure measurements did not affect the decision to proceed with DHC.
Similar, to the meta-analysis done by Vahedi [26] for patients who received DHC for malignant stroke that demonstrated a shift towards higher probability of having a mRS of 4 in the surgical group, we were concerned that intervening surgically on ICH patients may shift patients from a mRS of 6 to an mRS of 5. Although having improved mortality, this would still be considered a poor outcome to most. However, our results show relatively similar rates of a mRS 5 between the two cohorts (Table 2).
The efficacy of DHC in ICH in our study is similar to the results seen in other case and case-control series. The oldest and largest reported series of patients with DHC comes from a 73 patient casecontrol series by Dierssen et al. [20] ICH score was not available during this study, but 43 (59%) patients presented with a neurological exam of stuporous to deep coma. Mortality was 33% in this group and 45% of patients were reported to have good outcome. Despite having a poor initial presentation, the long-term functional outcome was good in nearly half of the survivors and they found a statistically significant improvement in mortality after craniectomy. Murthy et al. [15] reported on a 12 patient cases series that had a median ICH score of 3. Most of the patients (92%) survived to follow-up, which was 17 months, and good outcome, defined as a mRS of 0-3, was achieved in 55% of patients. Ma et al. [16] performed a case-control series of 38 patients. Controls were patients who received a hematoma evacuation alone. In unadjusted analysis, there was a 32% mortality rate in the DHC group compared to 43% in the control group (p = 0.26). There were significantly more patients with herniation, patients with IVH, and a higher ICH score in the DHC group than the control group. When adjusted for these variables, the odds ratios for 30day mortality were 0.12 (95% CI 0.02-0.64, P = 0.01) Moreover, good outcome (GOS 3-5) was 55% in DHC group versus 45% in the controls (p = 0.28) with an adjusted odds ratio for good outcome of 23.23 (95% CI 2.13-252.86, P = 0.01).
Although DHC is used for life threatening herniation, there are a number of immediate and delayed postoperative complications to be considered. Immediate complications include external herniation which can lead to cortical lacerations, contusions, and/or damage to surface blood vessels [32]. Delayed complications include hydrocephalus and subdural hygromas, which have been reported extensively [32-35]. There may be anomalies in CSF dynamics present in these patients which may explain the pathogenesis of the hygromas [35]. Other complications include infections, seizures, hemorrhages, and syndrome of the trephined. In this study 4 out of 17 (24%) patients in our surgical arm developed subdural hygromas, but only one of these patients required a surgical procedure to reduce the size of the hygroma. In addition, 12% of the patients developed a subdural hemorrhage, but these were asymptomatic. One patient developed a new asymptomatic intraparenchymal hemorrhage that was found on routine CT scanning.
There are multiple limitations to our study. The sample size is small and this could account for not observing a statistically significant difference in mortality or functional outcome. It is possible that the treatment effect of DHC is small and therefore would only be detected with a larger sample size. Another factor that may account for the observed results is the time to surgery was on average beyond 48 hours from the time of hemorrhage. While this is not surprising due to this time window being when peak perihematomal edema formation occurs, it is possible that earlier DHC has greater benefit. This is a retrospective case-control study, so we are limited about the inferences that can be made about causal relationships. Our study may be subject to selection bias since there was no protocol in place for deciding who received DHC. The study was performed at a single academic referral center, limiting the generalizability. We did not have data related to specific treatments of cerebral edema or intracranial hypertension, and Neuro-ICU course such as medical complications, which could have impacted the long-term outcome. This suggests the possibility of residual confounding, which may have influenced the association between case or control status (DHC) and outcome. However, we attempted to control for residual confounders by our matching methodology (ICH score and age). Our results should be interpreted with this in mind.
Conclusion
This case-control study suggests that in ICH patients with ICH score greater than 2; DHC may be life-saving in the long-term. Future prospective randomized controlled studies using a larger but selected patient population are warranted to assess the potential benefit of DHC after spontaneous ICH.
Acknowledgements
Drs. Shah, Bar, and Rincon had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conception and design: OS, BB
Acquisition of data: OS, BB
Analysis and interpretation: OS, BB, FR
Drafting the manuscript for important intellectual content: OS, BB, FR
Administrative, technical, or material support: BB
Study supervision: BB, FR
Statistical analysis: FR
Sources of Funding
Dr. Rincon has received salary support from the American Heart Association (AHA 12CRP12050342).
Dr. Shah has received grant support from the Society of Critical Care Medicine – PA Chapter.
Disclosures and Conflicts of Interest
Dr. Rincon: Philadelphia Representative SCCM-PA, Chairelect Neuroscience Section SCCM, and Board of Directors of the Neurocritcal Care Society.
References
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011; 123: e18-e209.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project--1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. Journal of neurology, neurosurgery, and psychiatry. 1990; 53: 16-22.
- Fred Rincon, Stephan A. Mayer. The epidemiology of intracerebral hemorrhage in the United States from 1979 to 2008. Neurocritical care. 2013; 19: 95-102.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke; a journal of cerebral circulation. 1993; 24: 987-93.
- Appelboom G, Piazza MA, Hwang BY, Carpenter A, Bruce SS, Mayer S, et al. Severity of intraventricular extension correlates with level of admission glucose after intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2011; 42: 1883-1888.
- Helweg-Larsen S, Sommer W, Strange P, Lester J, Boysen G. Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke; a journal of cerebral circulation. 1984; 15: 1045-1048.
- Giroud M, Gras P, Chadan N, Beuriat P, Milan C, Arveux P, et al. Cerebral haemorrhage in a French prospective population study. Journal of neurology, neurosurgery, and psychiatry. 1991; 54: 595-598.
- Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr., et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2010; 41: 2108-2129.
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005; 365: 387-397.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; 382: 397-408.
- Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke; a journal of cerebral circulation. 2013; 44: 627-634.
- Takeuchi S, Nawashiro H, Wada K, Takasato Y, Masaoka H, Hayakawa T, et al. Ventriculomegaly after decompressive craniectomy with hematoma evacuation for large hemispheric hypertensive intracerebral hemorrhage. Clinical neurology and neurosurgery. 2013; 115: 317-322.
- Shimamura N, Munakata A, Naraoka M, Nakano T, Ohkuma H. Decompressive hemi-craniectomy is not necessary to rescue supratentorial hypertensive intracerebral hemorrhage patients: consecutive single-center experience. Acta neurochirurgica Supplement. 2011; 111: 415-419.
- Ramnarayan R, Anto D, Anilkumar TV, Nayar R. Decompressive hemicraniectomy in large putaminal hematomas: an Indian experience. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2009; 18: 1-10.
- Murthy JM, Chowdary GV, Murthy TV, Bhasha PS, Naryanan TJ. Decompressive craniectomy with clot evacuation in large hemispheric hypertensive intracerebral hemorrhage. Neurocritical care. 2005; 2: 258-262.
- Ma L, Liu WG, Sheng HS, Fan J, Hu WW, Chen JS et al. Decompressive craniectomy in addition to hematoma evacuation improves mortality of patients with spontaneous basal ganglia hemorrhage. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2010; 19: 294-298.
- Kim KT, Park JK, Kang SG, Cho KS, Yoo DS, Jang DK, et al. Comparison of the effect of decompressive craniectomy on different neurosurgical diseases. Acta neurochirurgica. 2009; 151: 21-30.
- Heuts SG, Bruce SS, Zacharia BE, Hickman ZL, Kellner CP, Sussman ES, et al. Decompressive hemicraniectomy without clot evacuation in dominantsided intracerebral hemorrhage with ICP crisis. Neurosurgical focus. 2013; 34: E4.
- Fung C, Murek M, Z’Graggen WJ, Krahenbuhl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2012; 43: 3207-3211.
- Dierssen G, Carda R, Coca JM. The influence of large decompressive craniectomy on the outcome of surgical treatment in spontaneous intracerebral haematomas. Acta neurochirurgica. 1983; 69: 53-60.
- Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2001; 32: 891-897.
- Hemphill JC, 3rd, Farrant M, Neill TA, Jr. Prospective validation of the ICH Score for 12-month functional outcome. Neurology. 2009; 73: 1088-1094.
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007; 38: 1091-1096.
- Bruno A, Akinwuntan AE, Lin C, Close B, Davis K, Baute V, et al. Simplified modified rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke. 2011; 42: 2276-2279.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007; 147: 573-577.
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet neurology. 2007; 6: 215-222.
- Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressivehemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurgical focus. 2013; 34: E5.
- Dorfer C, Frick A, Knosp E, Gruber A. Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage. World neurosurgery. 2010; 74: 465- 471.
- Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. The New England journal of medicine. 2011; 364: 1493-1502.
- Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2001; 17: 154-162.
- Hanley D, editor Mistie Trial: 365-day Results Demonstrate Improved Outcomes and Cost Benefit. International Stroke Conference; 2013; Honolulu, HI.
- Chu SY, Sheth KN. Decompressive craniectomy in neurocritical care. Curr Treat Options Neurol. 2015; 17: 330.
- Aarabi B, Chesler D, Maulucci C, Blacklock T, Alexander M. Dynamics of subdural hygroma following decompressive craniectomy: a comparative study. Neurosurgical focus. 2009; 26: E8.
- De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M, et al. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: a timeline of events? Clinical neurology and neurosurgery. 2013; 115: 1308- 1312.
- Takeuchi S, Takasato Y, Masaoka H, Nagatani K, Otani N, Wada K, et al. Decompressive craniectomy for arteriovenous malformation-related intracerebral hemorrhage. J Clin Neurosci. 2015; 22: 483-487.