
Mini Review
Austin Neurol & Neurosci. 2016; 1(2): 1009.
Novel Insights for Therapy of Parkinson’s disease: Pharmacological Modulation of the Ca2+/cAMP Signalling Interaction
Bergantin LB* and Caricati-Neto A
Department of Pharmacology, Universidade Federal de Sao Paulo, Escola Paulista de Medicina, Laboratory of Autonomic and Cardiovascular Pharmacology, Sao Paulo, Brazil
*Corresponding author: Leandro Bueno Bergantin, Department of Pharmacology, Universidade Federal de Sao Paulo, Escola Paulista de Medicina, Laboratory of Autonomic and Cardiovascular Pharmacology, Sao Paulo, Brazil
Received: August 26, 2016; Accepted: October 18, 2016; Published: October 21, 2016
Abstract
Our discovery of the “calcium paradox” phenomenon due to interaction between Ca2+/cAMP intracellular signalling pathways involved in catecholaminergic transmission may provide new insights for the treatment of psychiatric disorders, such as Parkinson’s disease. This disease is mainly resulting by reduction of dopamine release in striatal dopaminergic neurons. In addition, since 1975 several clinical studies have reported that administration of L-type Ca2+ Channel Blockers (CCBs) in hypertensives produces reduction in vascular resistance and arterial pressure, associated with an increase in plasma noradrenaline levels and tachycardia characterized by sympathetic hyperactivity. Despite these adverse effects of CCBs have been initially attributed to adjust reflex of arterial pressure, during almost four decades these enigmatic phenomena remained unclear. In 2013, we discovered that this paradoxical sympathetic hyperactivity produced by CCBs is due to interaction of the Ca2+/cAMP intracellular signalling pathways. Also, clinical studies have been reporting neuroprotective effects of CCBs in neurodegenerative disorders, including for Parkinson’s disease. The molecular mechanisms involved in these pleiotropic effects remain under debate. Then, the pharmacological manipulation of the Ca2+/cAMP interaction could be a more efficient therapeutic strategy for increasing neuroprotection and dopamine neurotransmitter release in Parkinson’s disease.
Keywords: Parkinson’s disease; Ca2+/cAMP interaction
Introduction
Parkinson’s disease is a neurodegenerative disease resulting mainly by reduction of dopamine release from striatal dopaminergic neurons due to neuronal death [1]. Neurodegeneration in Parkinson’s disease begins years before a clinical diagnosis can be consistently made (asymptomatic/slightly symptomatic patients). The early diagnostic phase of the disease offers an opportunity for therapies, for example: those aimed to interrupt or preventing the progression of this disease, and its many complications side effects, could be more beneficial, but no such efficient therapies are available at the present moment. Thus, revealing the mechanisms of neurodegeneration from the earliest stages, however, could lead to the development of new interventions, whose therapeutic potential will need to be assessed in adequately designed clinical trials [1]. Advances in the understanding of this early phase of Parkinson’s disease will lead to the identification of biomarkers of neurodegeneration and its progression. These biomarkers will help to identify the ideal population to be included, and the most appropriate outcomes to be assessed in clinical trials of medicines. Potential risks for asymptomatic patients developing Parkinson’s disease, and individuals who do not wish to know their mutation status, could pose specific ethical dilemmas in the design of clinical trials. In this chapter, we discuss novel strategies to treat Parkinson’s disease, throughout our recent discovery entitled “calcium paradox” phenomenon due to interaction of Ca2+/cAMP intracellular signalling pathways [2-4].
Current Therapy to Treat Parkinson’s Disease
Dopamine loss in the substantia nigra, which results from reduction of dopamine release in striatal dopaminergic neurons due to neuronal death, outcomes in the recognizable core signs of asymmetrical bradykinesia and hypokinesia (slowness and reduced amplitude of movement), muscle rigidity (stiffness) and rest tremor, consequences from modifying motor control. Rest tremor, prominent asymmetry and a good response to levodopa are the features that most accurately predict Parkinson’s disease pathology [5]. The tremor-dominant form of Parkinson’s disease tends to run a more benign course than typical Parkinson’s disease. Early falls or autonomic symptoms, and a response to Parkinson’s disease medicines should raise evidences about the diagnosis [5]. Medicationinduced Parkinsonism due to commonly prescribed dopamineblocking medications, such as antipsychotics (eg: haloperidol, risperidone) and antiemetics (eg: metoclopramide, prochlorperazine) should be excluded in Parkinson’s patients. Functional imaging of the dopaminergic system using cerebral single photon emission computed tomography or positron emission tomography can be useful in diagnosis of early Parkinson’s disease [1,5]. Positron emission tomography studies examining the rate of decline in dopamineproducing cells suggest that humans have already lost 50%-70% of their nigral neurons, before they develop motor symptoms [5], and it has been estimated that the duration of this “presymptomatic” phase is about 5 years. Early diagnosis will become a critical issue if effective neuroprotective drugs become available. In fact, increasing dopamine, mainly by Levodopa combined with a dopa-decarboxylase inhibitor remains the most potent drug therapy for reversing motor impairment. A higher maintenance dose of Levodopa (eg: 200 mg three times daily compared with an initial dose of 100 mg three times daily) provides slightly greater benefit for reducing motor symptoms, but at the cost of earlier wearing-off symptoms and dyskinesias [5]. The combination of novel concepts may lead to advances in Parkinson’s disease research with the promise of finding compounds that are both effective, and fast-acting, including in patients who have tried other therapies with limited success. In conclusion, new insights for more efficient pharmacological treatments of Parkinson’s disease are clearly needed.
Novel Insights for Therapy of Parkinson’s Disease: Pharmacological Modulation of the Ca2+/cAMP Signalling Interaction
Discovery of the role of interaction of intracellular signalling pathways mediated by Ca2+ and cAMP in neurotransmitter release: A brief review
Numerous experiments initiated sixty years ago using catecholaminergic cells originated the concept of stimulus-secretion coupling to elucidate neurotransmitter release and hormone secretion. This concept was initially resulted from the study of cat adrenal gland perfused with acetylcholine executed by Douglas and Rubin in the 1960’s [6]. The discovery that increase in the cytosolic Ca2+ concentration ([Ca2+]c) was a basic requirement for exocytosis in adrenal catecholaminergic cells was made by Baker and Knight in 1970’s [7]. In addition, some studies showed that cAMP rises transmitter release at several synapses in autonomic nervous system of vertebrate, including sympathetic neurons [8]. Although the cellular and molecular mechanisms involved in these synergistic actions of cAMP on the exocytosis of neurotransmitter and hormones remain uncertain, the evidences suggest that this intracellular messenger can participate in fine regulation of exocytosis due to its modulatory action on the intracellular Ca2+ signals.
In fact, the hypothesis for an interaction between the intracellular signalling pathways mediated by Ca2+ and cAMP (Ca2+/cAMP interaction) has been extensively studied in many cells and tissues. Generally, this interaction results in synergistic effects on cell functions [2-4] and occurs at the level of Adenylyl Cyclases (ACs) or Phosphodiesterases (PDEs) (Figure 1). The Ca2+/cAMP interaction has particularly been extensively studied at the Ca2+ channels [e.g: Ryanodine Receptors (RyR)] of the Endoplasmic Reticulum (ER) [2-4]. Phosphorylation of RyR by Protein Kinase A (PKA), and also Inositol trisphosphate receptor (IP3R) at submaximal IP3 concentrations, may increase the open probability of ER Ca2+ stores, amplifying Ca2+-Induced Ca2+ Release (CICR) mechanism and cellular responses [2-4] (Figure 1). Then, dysfunctions of cellular homeostasis of Ca2+ and/or cAMP in these cells could result in the dysregulation of Ca2+/cAMP interaction and exocytotic response, or could be a novel therapeutic target for medicines (Figure 1).
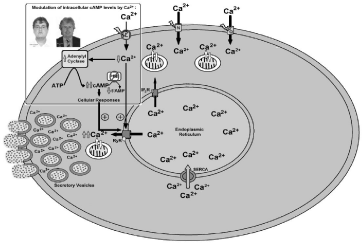
Figure 1: Role of Ca2+/cAMP interaction in neurotransmitter release, including dopamine from central nervous system.
Cellular homeostasis of Ca2+ and/or cAMP in these cells could be a novel therapeutic target for medicines, according to our previous studies [2-4]. Considering our model in which increment of [cAMP]c stimulates Ca2+ release from endoplasmic reticulum, it may be plausible that the therapeutic use of the PDE inhibitor rolipram in combination with low doses of verapamil to potentiate neurotransmission in the areas of central nervous system involved in neurological/psychiatric disorders in which neurotransmission is reduced, including Parkinson’s disease.
Paradoxical effects of CCBs on neurotransmission and their pleiotropic effects in Parkinson’s disease
Since four decades ago, several clinical studies have been reporting that acute and chronic administration of L-type Ca2+ Channel Blockers (CCBs), such as nifedipine and verapamil, produces reduction in peripheral vascular resistance and arterial pressure associated with an increase in plasma noradrenaline levels and heart rate, typical effects of sympathetic hyperactivity [9]. However, the cellular and molecular mechanisms involved in this apparent sympathomimetic effect of the L-type CCBs remained unclear for decades. In addition, experimental studies using isolated tissues richly innervated by sympathetic nerves showed that neurogenic responses were completely inhibited by L-type CCBs in high concentrations (>1 μmol/L), but paradoxically potentiated in concentrations below 1 μmol/L [10-12]. During almost four decades, these enigmatic phenomena remained unclear. In 2013, we discovered that this paradoxical increase in sympathetic activity produced by L-type CCBs is due to Ca2+/cAMP interaction [2-4]. Then, the pharmacological manipulation of the Ca2+/cAMP interaction produced by combination of the L-type CCBs used in the antihypertensive therapy, and cAMP accumulating compounds used in the anti-depressive therapy such as rolipram, could represent a potential cardiovascular risk for hypertensive patients due to increase in sympathetic hyperactivity. In contrast, this pharmacological manipulation could be a new therapeutic strategy for increasing neurotransmission in the psychiatric disorders, such as Parkinson’s disease.
In addition, several studies have been demonstrating pleiotropic effects of CCBs. CCBs, like nifedipine, genuinely have pleiotropic effects [13]. Ca2+ channels are important regulators of central nervous system, and their dysfunction can give rise to pathophysiological conditions as psychiatric conditions such as epilepsy, pain and autism [13]. In the nervous system, CCBs have been emerging as potential therapeutic avenues for pathologies such as Parkinson’s disease [13]. However, the molecular mechanisms involved in these pleiotropic effects remain under debate. Different mechanisms have been proposed, but the exact mechanisms are still uncertain.
Importance of pharmacological modulation of Ca2+/cAMP interaction in the treatment of Parkinson’s disease and other neurodegenerative diseases
In contrast to adverse effects produced by combination of L-type CCBs with cAMP accumulating compounds in the cardiovascular diseases, the pharmacological implications of the Ca2+/cAMP interaction produced by this drug combination could be used to enhance neurotransmission [2-4].
Considering our model in which increment of [cAMP]c stimulates Ca2+ release from ER (Figure 1), it may be plausible that the therapeutic use of the PDE inhibitor rolipram [14,15], in combination with low doses of verapamil to increase neurotransmission (Figure 1) in the areas of central nervous system involved in neurological/ psychiatric disorders in which neurotransmission is reduced, including Parkinson’s disease. This new pharmacological strategy for the treatment of psychiatric disorders could increase the therapeutic efficacy and reduce the adverse effects of the medicines currently used for treating Parkinson’s disease. Considering that CCBs genuinely exhibit cognitive-enhancing abilities and reduce the risk of neurodegenerative diseases like Parkinson’s disease [13]; and that the mechanisms involved in these pleiotropic effects are largely unknown. Then, whether Ca2+/cAMP interaction is involved in such effects deserves special attention.
In addition, considering [Ca2+]c elevation could contribute to both: negatively to neuroprotective effects and positively to exocytosis, it may be plausible the therapeutic use of the PDEs inhibitors [14,15] for antiparkinsonism purposes. Then, pharmacological interference of the Ca2+/cAMP interaction produced by combination of L-type CCBs and cAMP-accumulating compounds could enhance antiparkinsonism response and reduce clinical symptoms of neurodegenerative diseases. Thus, the association of currently medicines could enhance antiparkinsonism treatments. For example: the association of Levodopa with CCBs or rolipram could dramatically improve typical antiparkinsonism medicines, mainly by reducing their adverse effects and increasing their effectiveness. This new pharmacological strategy could be alternatively used for treatment of the symptoms of neurodegenerative diseases.
Conclusion
The diagnosis of neurodegenerative diseases like Parkinson’s disease relies critically on clinical diagnosis of patients. In addition, emerging therapies may supplement clinical assessment in the next years. Although pharmacological therapies have been largely unsuccessful in attenuating Parkinson’s disease symptoms, targeting potential risk factors aiming to decrease incidence of this neurodegenerative disease is an important public health issue. Finally, novel strategies to treat Parkinson’s diseases, throughout our recent discovery entitled “calcium paradox” phenomenon due to Ca2+/cAMP interaction, could greatly contribute to enhance therapeutic strategies for increasing neuroprotection. Thus, the association of typical antiparkinsonism medicines with CCBs or rolipram could dramatically improve antiparkinsonism therapies, mainly by reducing adverse effects and improving effectiveness of these currently medicines [16].
References
- Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson's disease. Lancet Neurol. 2016; 15: 637-648.
- Caricati-Neto A, Garcia AG, Bergantin LB. Pharmacological implications of the Ca2+/cAMP signalling interaction: from risk for antihypertensive therapy to potential beneficial for neurological and psychiatric disorders. Pharmacol Res Perspect. 2015; 3: e00181.
- Bergantin LB, Souza CF, Ferreira RM, Smaili SS, Jurkiewicz NH, Caricati-Neto A, et al. Novel model for “calcium paradox” in sympathetic transmission of smooth muscles: role of cyclic AMP pathway. Cell Calcium. 2013; 54: 202-212.
- Bergantin LB, Jurkiewicz A, Garcia AG, Caricati-Neto A. A Calcium Paradox in the Context of Neurotransmission. Journal of Pharmacy and Pharmacology. 2015; 3: 253-261.
- Hayes MW, Fung VS, Kimber TE, O'Sullivan JD. Current concepts in the management of Parkinson disease. Med J Aust. 2010; 192: 144-149.
- Douglas WW, Rubin RP. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961; 159: 40-57.
- Baker PF, Knight DE. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978; 276: 620-622.
- Chern YJ, Kim KT, Slakey LL, Westhead EW. Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem. 1988; 50: 1484-1493.
- Grossman E, Messerli FH. Effect of calcium antagonists on sympathetic activity. Eur Heart J. 1998.
- Kreye VA, Luth JB. Proceedings: verapamil-induced phasic contractions of the isolated rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1975.
- French AM, Scott NC. A comparison of the effects of nifedipine and verapamil on rat vas deferens. Br J Pharmacol. 1981; 73: 321-323.
- Moritoki H, Iwamoto T, Kanaya J, Maeshiba Y, Ishida Y, Fukuda H. Verapamil enhances the non-adrenergic twitch response of rat vas deferens. Eur J Pharmacol. 1987; 140: 75-83.
- Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2016; 15: 19-34.
- Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O'Donnell, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011; 31: 172-183.
- Xiao L, O'Callaghan JP, O'Donnell JM. Effects of Repeated Treatment with Phosphodiesterase-4 Inhibitors on cAMP Signaling, Hippocampal Cell Proliferation, and Behavior in the Forced-Swim Test. J Pharmacol Exp Ther. 2011; 338: 641-647.
- Bergantin LB, Caricati-Neto A. Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca2+/cAMP intracellular signalling interaction. European Journal of Pharmacology. 2016; 788: 255-260.