
Review Article
Austin Neurol & Neurosci. 2016; 1(3): 1011.
The Role of Infiltrating Monocytes/Macrophages in Intracerebral Hemorrhage
Sun J and Nan G*
Department of Neurology, China-Japan Union Hospital of Jilin University, China
*Corresponding author: Guangxian Nan, Department of Neurology, China-Japan Union Hospital of Jilin University, China
Received: August 19, 2016; Accepted: October 30, 2016; Published: November 03, 2016
Abstract
Intra Cerebral Hemorrhage (ICH), a subtype of stroke, pose a serious threat to human life. Recent studies have shown that neuroinflammation is deeply related to the progression of ICH-induced brain injury. After the onset of ICH, peripheral circulatory system monocytes/macrophages can be activated within hours and recruited to the peri-hematoma regions. Traditionally, activation of monocytes/macrophages is considered to play a deleterious role in cerebral hemorrhage, as inhibition of their activation attenuates hemorrhage-induced brain injury. However, increasing evidence shows that activation of monocytes/ macrophages is critical for hematoma clearance and functional recovery after ICH. Therefore, a better understanding of the mechanisms underlying their functional changes following ICH is necessary. We briefly review the activation, function, and phenotypes of monocytes/macrophages after ICH and then suggest additional therapies targeting monocytes/macrophages that may be aim at not only suppressing their activation, but also modulating them at different stages of ICH. However, more work is needed to elucidate the cellular and molecular mechanisms of infiltrating monocyte differentiation and macrophage polarization in a hemorrhagic brain environment.
Keywords: Intracerebral hemorrhage; Monocytes; Macrophages; Polarization
Introduction
Intra Cerebral Hemorrhage (ICH) is a devastating stroke subtype affecting nearly 2 million patients all over the word every year. While the initial neurological deficit is caused by the mass effect of the hemorrhage itself, there is increasing recognition that an inflammatory process contributes to further injury over the ensuing days [1-3]. After ICH, extravasation of plasma protein and cellular elements from the blood vessels into the brain tissue represents the triggering factor for the mobilization and activation of the brain immune cells. As a result, microglia, astrocytes and endothelium cells secrete inflammatory cytokines and chemokines, which induce robust recruitment of leukocytes from blood into the peri-hematoma region within hours to a few days, such as monocytes/macrophages, neutrophils and lymphocytes.
Among these circulating immune cells, monocytes/macrophages have been shown to play a particularly important role. Initially, the presence of monocytes/macrophages at the injury region was considered as a marker of an exacerbated inflammatory response that contributed to brain injury. However, recent studies show that infiltrating monocytes/macrophages in the brain post-ICH contribute to functional recovery, implying a protective effect of these cells after ICH [4-6]. These reports suggest a more complex and multiphase role of infiltrating monocytes/macrophages, suggesting a potential direction for ICH therapies.
Activation of monocytes/macrophages in ICH
Monocytes constitute a heterogeneous group of cells, including an inflammatory subset and patrolling subset. The inflammatory monocytes are specifically recruited to an injury site and differentiated into macrophages. The patrolling subsets are recruited to normal tissue and participate in host defense and repair after injury. After tissue injury, early inflammatory monocytes and late patrolling monocytes are sequentially recruited to the lesion in a controlled manner for inflammation and repair/healing [7].
Following ICH, the inflammatory monocytes migrate into and around the lesion site as soon as possible and then differentiate into potent phagocytic cells, macrophages, in the hemorrhagic brain. Actually, inflammatory monocytes differentiate into macrophages in the hemorrhagic brain as early as 12 hours after ICH and can be seen for a long time in the brain, even months [8]. It is reported that inflammatory monocytes are the most numerous blood-derived leukocytes in the brain at day 3 after experimental ICH [9]. In recent hemorrhages (within 5-10 days after stroke), a large number of the macrophage system cells around the hemorrhage core originate from the blood flow by activation of circulating monocytes, while a lesser number result from local microglia activation [10].
After ICH, the migration of monocytes/macrophages is correlated to many factors, such as neutrophils and inflammation-induced high expression of chemokine and chemokine receptors. It has been reported that up-regulation of Monocyte Chemo attractant Protein- 1(MCP-1) and its receptor CC chemokine Receptor 2 (CCR2) could induce greater accumulation of monocytes/macrophages and exacerbate inflammatory injury in the hemorrhagic brain [11]. However, there is a relative paucity of in vivo studies related to the activity of patrolling monocytes following ICH.
Function of monocytes/macrophages in ICH
In a rat model of ICH, robust blood-derived inflammatory monocytes are recruited to the region surrounding the hematoma and contribute to early motor deficits [8]. Besides, a higher serum CCL2 level, the dominant chemokine for monocyte recruitment, at 24 h is also associated with worse early functional outcomes in patients with ICH [9]. Lee et al. showed that a splenectomy 3 days prior to ICH rescued brain edema and infiltrating macrophages and neutrophils [10,11], further implicating the role of peripheral macrophages in ICH damage [12]. In addition, the peripheral monocyte count is also associated with 30-day case-fatality in ICH patients [13,14]. These studies suggest that peripheral monocytes/macrophages migrate to the hematoma region and then lead to progressive brain damage, and inhibition of their trafficking into the brain or a reduction in the peripheral monocytes/macrophages may have a therapeutic benefit in ICH.
However, studies have also proven that monocytes/macrophages infiltrating brain parenchyma after ICH may play a beneficial role in clearance of the hematoma, anti-inflammation and repair as well as promote recovery from ICH injury [4,5]. Zhao et al. reported that macrophages play a key role in promoting hematoma absorption and protecting other brain cells from ICH-induced injury via PPARγ [15]. After hemorrhage, M2 macrophage are expected to increase over time as the peri-hematoma milieu transitions into an immune dampened tissue repair phage in which M2 macrophage play a pivotal role [16]. Besides, it’s also proved that CD163, mainly localized on the surface of monocytes/macrophages, counteract the inflammation and promote hematoma absorption as well as improves neurological functions in patients with intra cerebral hemorrhage [17,18]. Recently, in peripheral monocyte/macrophage-depleted mice, ICH was shown to induce a larger brain lesion volume and a more severe neurological deficit than those in control mice at day 3 post-ICH, suggesting a protective role of monocytes/macrophages in ICH [5].
Considering these evolutionarily adaptive functions, we believe that infiltrating monocytes/macrophages play a critical role in the innate immune response after ICH. On the one hand, they play a deleterious role via releasing a variety of inflammatory cytokines and toxic substances. On the other hand, they could also be beneficial through clearance of hematoma and repair. Therefore, a better understanding of the mechanisms underlying the functional changes of monocytes/macrophages in the pathobiology of ICH is essential to develop successful immune interventions. Maybe different phenotypes of monocytes/macrophages could account for their biphasic roles under different pathological conditions.
Phenotypes of monocytes/macrophages in ICH
It is widely accepted that there are two types of macrophages: classically activated macrophages (M1) and alternatively activated macrophages (M2). M1 cells express high levels of CD86, CD16, CD32 and MHCII on their surface and produce large amounts of pro-inflammatory cytokines, such as TNF-α, IL-6, iNOS and NO, exacerbating tissue damage. M2 cells have low levels of MHCII and CD86 and poorly stimulate or even inhibit T cell proliferation but express a number of protein important for pinocytosis of carbohydrate-rich parasitic products and promote tissue repair [19]. It is well known that macrophages display plasticity in their characteristics and can change phenotype and function depending on the microenvironment.
It is accepted that M2 macrophages co-exist with M1 macrophages throughout the course of disease development, although M2 cells are not the predominant macrophage phenotype at the initial phase. There is a gradual increase in the population of M2 cells during the process of inflammation until the peak of disease, whereas the M1 cell population is relatively reduced in the later phases of disease development [20]. An increase in the M2 cell population in the peak and later stages of disease may contribute to a decrease in inflammation by expressing anti-inflammatory cytokines. Thus, the balance between macrophage phenotypes is important in disease development, and a disorder of balance may be either detrimental or beneficial to disease progress. Breakthrough research on macrophages has revealed several transcriptional regulators that serve as central switches to turn on a group of M1 or M2 genes and achieve polarization. The most studied are the IRF/STAT, JNK and Akt/PI3 signaling pathways [21,22]. In the recent years, different studies note that certain miRNAs are also involved in the acquisition of the M1 and M2macrophageactivated states, such as miRNA155 and miRNA124 [19,23]. In addition, the existence of other cells may also affect the phenotype of macrophages, such as glia and neural stem cells [5,24].
In ICH, the microenvironment greatly influences the phenotypic changes in macrophages, resulting in different gene expression patterns and bio functions in the same brain tissue. Currently, there is a general lack of information about the time course of M1 and M2 macrophage activation. Yang et al. found that M2 markers (Arg 1, IL- 13, YM 1, and CD206) increased within 1 day, but the M1 markers (IL- 1β, TNF-α, and IL-6)increased as early as 3 h after ICH [25]. They also showed that early injection of IL-4 could promote anti-inflammation, which favors repair via inhibiting M1 cell activation while enhancing M2 cell activation after experimental ICH. This suggests that drugs with the ability to promote the shift to the M2 phenotype might be beneficial for the treatment of ICH. In addition, other peripheral immune cells may also affect macrophage phenotypes after ICH. For instance, it has been shown that regulatory T cells can induce macrophages towards M2 polarization through the IL-10/GSK3β / PTEN axis [26].
Circulating monocytes possess 3 major distinct phenotypes in human and mice based on the expression of specific surface markers. The phenotype(CD14+CD16- in humans and Ly6ChiCD43- in mice) expressing a high level of CCR2 is the classical monocytes, which can migrate to the site of injury and infection where they differentiate into macrophages; the phenotype (CD14dimCD16+ in humans and Ly6CloCD43+ in mice) expressing a high level of CX3CR1 is the non classical patrolling monocytes, which exhibit a unique ability to patrol the resting vasculature and remove debris; a third phenotype (CD14+CD16+ in humans and Ly6ChiCD43+ in mice) with high expression of CX3CR1 is the intermediate monocytes, which also generally possess inflammatory characteristics [27]. It has been reported that the Ly6Clomonocytes recruited during inflammation only differentiate into M2 macrophages, while Ly6Chimonocytespolarize to M1 macrophages in the early phase of inflammation but polarized to M2 macrophages in the later phage when the inflammation was receding in renal tubulointerstitial injury [28,29]. It has also been reported that PPAR? activation primes monocyte differentiation into M2 macrophages in human atherosclerotic lesions [30]. However, the exact contribution of different monocyte phenotypes in ICH is still elusive as studies related to monocyte differentiation and its influential factors following ICH are limited (Figure 1).
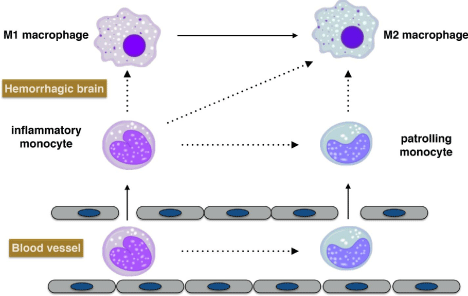
Figure 1: Potential lineage relationships of monocytes and macrophages
after ICH. In steady-state, monocyte conversion from inflammatory subset
to patrolling subset may occur in blood and bone marrow [27]. It is generally
accepted that inflammatory monocytes give rise to M1 macrophages in
tissue. Other lineage relationships, indicated by dashed arrows, remain to be
experimentally explored in the condition of intracerebral hemorrhage.
Circulating monocytes possess 3 major distinct phenotypes in human and mice based on the expression of specific surface markers. The phenotype(CD14+CD16- in humans and Ly6ChiCD43- in mice) expressing a high level of CCR2 is the classical monocytes, which can migrate to the site of injury and infection where they differentiate into macrophages; the phenotype (CD14dimCD16+ in humans and Ly6CloCD43+ in mice) expressing a high level of CX3CR1 is the non classical patrolling monocytes, which exhibit a unique ability to patrol the resting vasculature and remove debris; a third phenotype (CD14+CD16+ in humans and Ly6ChiCD43+ in mice) with high expression of CX3CR1 is the intermediate monocytes, which also generally possess inflammatory characteristics [27]. It has been reported that the Ly6Clomonocytes recruited during inflammation only differentiate into M2 macrophages, while Ly6Chimonocytespolarize to M1 macrophages in the early phase of inflammation but polarized to M2 macrophages in the later phage when the inflammation was receding in renal tubulointerstitial injury [28,29]. It has also been reported that PPAR? activation primes monocyte differentiation into M2 macrophages in human atherosclerotic lesions [30]. However, the exact contribution of different monocyte phenotypes in ICH is still elusive as studies related to monocyte differentiation and its influential factors following ICH are limited (Figure 1).
References
- Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014; 8: 388.
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014; 115: 25-44.
- Chen S, Yang Q, Chen G, Zhang JH. An Update on Inflammation in the Acute Phase of Intracerebral Hemorrhage. Translational Stroke Research. 2015; 6: 4-8.
- Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009.
- Min H, Jang YH, Cho IH, Yu SW, Lee SJ. Alternatively activated brain-infiltrating macrophages facilitate recovery from collagenase-induced intracerebral hemorrhage. Mol Brain. 2016; 9: 42.
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization -new prospects for brain repair. Nat Rev Neurol. 2015; 11: 56-64.
- Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014; 34: 1411-1419.
- Hammond MD, Ai Y, Sansing LH. Gr1+ macrophages and Dendritic Cells Dominate the Inflammatory Infiltrate 12 Hours After Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2012.
- Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, et al. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014; 34: 3901-3909.
- Dahnovici RM, Pintea IL, Malaescu DG, Busuioc CJ, Predescu A, Mogoanta L. Microscopic aspects of macrophage system cells in hemorrhagic stroke in humans. Rom J Morphol Embryol. 2011; 52: 1249-1253.
- Yao Y, Tsirka SE. Chemokines and their receptors in intracerebral hemorrhage. Transl Stroke Res. 2012; 3: 70-79.
- Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008; 131: 616-629.
- Adeoye O, Walsh K, Woo JG, Haverbusch M, Moonaw CJ, Broderick JP, et al. Peripheral monocyte count is associated with case-fatality after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014.
- Walsh KB, Sekar P, Langefeld CD, Moomaw CJ, Elkind MS, Boehme AK, et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke. 2015; 46: 2302-2304.
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007; 61: 352-362.
- Klebe D, McBride D, Flores JJ, Zhang JH, Tang J. Modulating the Immune Response Towards a Neuroregenerative Peri-injury Milieu After Cerebral Hemorrhage. J Neuroimmune Pharmacol. 2015; 10: 576-586.
- Liu B, Hu B, Shao S, Wu W, Fan L, Bai G, et al. CD163/Hemoglobin Oxygenase-1 Pathway Regulates Inflammation in Hematoma Surrounding Tissues after Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis. 2015; 24: 2800-2809.
- Xie WJ, Yu HQ, Zhang Y, Liu Q, Meng HM. CD163 promotes hematoma absorption and improves neurological functions in patients with intracerebral hemorrhage. Neural Regen Res. 2016; 11: 1122-1127.
- Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013; 61: 91-103.
- Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol Lett. 2014; 160: 17-22.
- Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014; 5: 614.
- Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Progress in Neurobiology. 2015; 131: 65-86.
- Wei Y, Schober A. MicroRNA regulation of macrophages in human pathologies. Cell Mol Life Sci. 2016; 73: 3473-3495.
- Gao J, Grill RJ, Dunn TJ, Bedi S, Labastida JA, Hetz RA, et al. Human Neural Stem Cell Transplantation-Mediated Alteration of Microglial/Macrophage Phenotypes after Traumatic Brain Injury. Cell Transplant. 2016.
- Yang J, Ding S, Huang W, Hu J, Huang S, Zhang Y, et al. Interleukin-4 Ameliorates the Functional Recovery of Intracerebral Hemorrhage Through the Alternative Activation of Microglia/Macrophage. Front Neurosci. 2016; 10: 61.
- Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis. J Cereb Blood Flow Metab. 2016.
- Zhu YP, Thomsa GD, Hedrick CC. 2014 Jeffrey M. Hoeg Award Lecture: Transcriptional Control of Monocyte Development. Arterioscler Thromb Vasc Biol. 2016; 36: 1722-1733.
- Lin SL, Castano AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009; 183: 6733-6743.
- Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011; 121: 3425-3441.
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007; 6: 137-143.
- Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011; 111: 173-178.
- Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. AnnNeurol. 2011; 70: 646-656.
- Zhao F, Hua Y, He Y, Keep RF, Xi G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011; 42: 3587-3593.