
Review Article
Austin Neurol & Neurosci. 2016; 1(3): 1013.
A Diverse Role of MMP-2 and MMP-9 in the Onset of Alzheimer Disease and Cancer
Chowdhury A*
Department of Biological Regulation, Weizmann Institute of Science, Israel
*Corresponding author: Animesh Chowdhury, Department of Biological Regulation, Weizmann Institute of Science, Israel, Email: animeshchowdhury9@gmail. com
Received: November 11, 2016; Accepted: December 08, 2016; Published: December 12, 2016
Abstract
Matrix Metallo Proteinases (MMPs) play a crucial role as inflammatory components in the pathogenesis of Alzheimer Disease (AD) and cancer. Among the MMPs, MMP-2 and MMP-9 has been considered as the most important enzymes due to their role in pathophysiology of diseases. An over expression of MMP-2 and MMP-9 has been observed to be associated with the progression of various types of cancer. An increase level of MMP-2 and MMP-9 has also been observed in the serum level of AD patients. In contrary, recombinant human MMP-2 and MMP-9 has been shown to degrade the Amyloid beta (Aβ) leading to the formation of a nontoxic soluble fragment of Aβ. Amyloid beta (Aβ) deposition is the main characteristic on the onset of AD. Therefore, over expression of MMP-2 and MMP-9 may lead to brain tumorigenesis, however, there could be reduced risk of AD.
Keywords: Alzheimer disease; Cancer, MMP-2, MMP-9, β-amyloid
Introduction
Matrix Metallo Proteinases (MMPs) are a family of highly homologous Zn2+-dependent enzymes, capable of degrading the extracellular matrix components. They are present in the normal cells and have been shown to play an important role in physiological processes such as wound healing, bone resorption and pregnancy [1- 3]. Among the MMPs, MMP-2 and MMP-9 has widely been accepted as the most valuable MMP in the pathogenesis of Alzheimer’s disease and cancer.
Neurodegenerative diseases are described as hereditary (caused by genetically) and sporadic (caused by environmental factor) conditions which are characterized by gradual dysfunction of nervous system consequently in the loss of memory and learning. These disorders are often related with atrophy of the affected central or peripheral structures of the nervous system which includes Alzheimer’s Disease (AD). AD is a progressive neurodegenerative disease characterized clinically by poor onset of memory and cognition impairment, emergence of psychiatric symptoms and behavioural disorders, and impairment of daily living activities. It is the most common form of dementia found in the elder mainly above 65 years. During the past decade, many hypotheses have been suggested for the pathogenesis of AD. Among them, the β-amyloid (Aβ) cascade has widely been accepted. One of the hallmarks of AD is the presence of senile plaques in the hippocampus formed by the deposition of Aβ, which is a 40-42 amino acid polypeptide.
Cancer is the second leading cause of death threatening disease worldwide. Cancer is a group of diseases associated with abnormal cell growth and potential to invade or spread to other parts of the body. However, not all tumors are cancerous; benign tumors are not malignant and do not invade nearby tissue and spread to other parts of the body. During the process of cancer invasion and metastasis, natural barriers such as interstitial connective tissue and basement membranes must have to be degraded. With the past two decades of biomedical research a huge amount of information on the molecular events that take place during carcinogenesis and the signalling molecules associated with the progression of cancer development have been represented. The molecular mechanisms of the complex interplay between the tumor cells and the tumor microenvironment play a crucial role in developing tumorigenesis related to cancer.
The aim of this article was to critically review the current status of MMP-2 and MMP-9 as prognostic markers in AD and cancer. Many experimental studies suggested that MMP-2/9 plays an important role in the progression of AD and cancer. Increased MMP-2/9 level in the serum have been shown to be associated with in AD and cancer patients case study. However, literatures also indicated the role of MMP-2/9 in the degradation of senile plaque, the most effective component found to lead AD progression. Therefore, MMP-2/9 could have diverse role in the onset of AD and cancer. This review will give the reader a background on the role of MMP-2 and MMP-9 associated with AD and various types of cancer and outlook of how this field can advance further.
Senile Plaques in AD
The main characteristic of AD is the formation of senile plaques which are polymorphous amyloid protein in nature. These plaques are generally found in large numbers in the limbic and association cortices [4]. Several reports have addressed the pathophysiological role of neurons, astrocytes, microglia, and capillaries in the development of senile plaques [5-9]. The size and morphology of senile plaques are highly variable and the size varies widely from 10 to 120 μm, and the density and degree of compaction of the amyloid fibrils which comprise the extracellular core also shows great variation among plaques [10]. These plaques are mainly formed by extracellular deposits of amyloid β-protein (Aβ) that occur primarily in a filamentous form, i.e., star-shaped masses of amyloid fibrils. Most of the fibrillar Aβ found in the senile plaques is the type ending at Amino acid 42 (Aβ42), slightly longer and more hydrophobic form potential to aggregate. However, the Aβ type ending at Amino acid 40 (Aβ40), which is generally more abundantly produced by cells than Aβ42, usually colocalized with Aβ42 in the plaque. The amyloid fibrils that compose the amyloid plaques are also constituted by different amounts of truncated forms of Aβ peptide, some of which derive from cleavage in the Aβ N-terminus to generate Aβ3–40/42, Aβ11– 40/42 and Aβ17–40/42; some from cleavage in the C-terminus to obtain Aβ1–39, Aβ1–38, and Aβ1–33; and some others from cleavage in both termini to produce Aβ25–35. Aβ25–35, among the other fragments, exhibits great properties of fibrillogenesis and toxicity similar to that of the intact Aβ, and is considered the biologically active region of Aβ [11].
Aβ-Degrading Enzymes
There is a kinetic equilibrium between production of Aβ, its degradation and transportation within the brain and transport out of the brain. Large number of evidences demonstrating the lack in the clearance of Aβ contributes to its accumulation leading to progression of AD. Numerous studies have suggested that Aβ peptide was degraded by a kind of protease called Aβ degrading enzyme. There are several numbers of this type of enzymes such as, Neprilysin (NEP), Endothelin Converting Enzyme (ECE) 1 and 2, Insulin Degrading Enzyme (IDE), Angiotensin-Converting Enzyme (ACE), plasmin and MMPs. All these protease elicits their function by degrading the Aβ peptide at different amino acid residues within the Aβ sequence. Injection of NEP inhibitor and/or NEP knockout mice showed decreased Aβ degradation and declined cognitive activities; on the other hand, over expression of NEP caused in improvement of spatial memory and decreased Aβ levels. Decrease in Aβ production is associated with decreased NEP activities, has been observed with increasing age and thus increase in Aβ degradation may have therapeutic importance. Similarly, several reports indicated the positive role of other Aβ degrading enzymes in the prevention of AD [12].
Role of MMP-2 and MMP-9 in AD
The functions of several MMPs to degrade Amyloid Precursor Protein (APP) leading to aggregation of Aβ, as well as the increased expression of MMPs has been shown to be associated with postmortem brain tissue of AD patients, suggesting that MMPs play an important role in the pathogenesis of AD. Among the MMPs MMP-9 has been shown to play the most important role in the pathogenesis of AD. Over expression of MMP-9 has been observed in the cytoplasm of neurons, neurofibrillary tangles, senile plaques, and vascular walls of hippocampus and cerebral cortex of AD patients [13-15]. A previous report also indicated the role of MMP-9 in pathogenesis of AD [16]. This study showed that an increase in circulating MMP-9 levels in plasma samples from AD patients as compared to controls indicating a possible role to the endothelial pathology of AD patients [16]. Romi et al. showed an increase level of MMP-9 level in the serum of AD patients [17]. Beside MMP-9, MMP-2 has also clinical importance in the pathogenesis of AD. Tamura et al demonstrated that MMP inhibitor II, which is reportedly highly selective for MMP- 2 and MMP-9, blocks Aβ-induced release of lactate dehydrogenase in primary cultured neurons, indicating that MMP-2 and MMP-9 contribute to Aβ-induced neuronal cell death [18]. Another report showed that the intra cerebroventricular injection of Aβ 25-35, Aβ1- 40, and Aβ1-42, but not Aβ40-1, transiently increased MMP-9, but not MMP-2, activity and protein expression in the hippocampus [19]. Immunohistochemistry revealed the expression of MMP-9 to be increased in both neurons and glial cells in the hippocampus after Aβ treatment [19]. The Aβ-induced cognitive impairment in vivo as well as neurotoxicity in vitro was significantly improved in MMP-9 homozygous knockout mice or by treatment with MMP inhibitors [20]. A recent study also showed significant increased expression levels of active MMP-2 in the entorhinal cortex at early stages of AD-related pathology [21]. However, there is controversy about the role of MMP- 2 and MMP-9 in pathogenesis of AD. A recent study demonstrated that neuronal over expression of MMP-9 in a transgenic AD mouse model harbouring five familial AD-related mutations (5xFAD) resulted in increased soluble APPa (sAPPa) levels and decreased Aβ oligomers without affecting amyloid plaque load in the brain [22]. Functionally, over expression of MMP-9 prevented the cognitive deficits displayed by 5xFAD mice accompanied by increased levels of the pre-synaptic protein synaptophysin and mature Brain-Derived Neurotrophic Factor (BDNF) in the brain [22]. Another study on AD found that MMP-9 was significantly decreased in serum [23]. MMP-2 might also be assumed to have a protective role in AD. A very recent study investigated the role of MMP-2 and MMP-9 in the degradation of Aβ [24]. In this study, recombinant human (rh) MMP-2 and MMP-9 were incubated with Aβ 40 and Aβ 42, and the resulting proteolytic fragments were examined by immunoprecipitation and quantitative mass spectrometry. Both MMP-2 and MMP-9 generated Aβ fragments truncated only at the C terminus, ending at positions 34, 30, and 16. Using deuterated homologues as internal standards, the researchers observed limited and relatively slow degradation of Aβ 42 by rhMMP-2, although the enzyme cleaved>80% of Aβ 40 during the 1st h of incubation. rhMMP-9 was comparatively less effective, particularly in degrading Aβ (1–42), although the targeted peptide bonds were identical. The researchers also revealed using Aβ (1–34) and Aβ (1–30) that these peptides were also substrates for both MMP-2 and MMP-9, and they cleave Aβ (1–34) to produce Aβ (1– 30) first and finally Aβ (1–16). Consistent with the kinetics observed with full-length Aβ, rhMMP-9 degraded only a minute fraction of Aβ (1–34) and was even less effective in producing Aβ (1–16). Additional degradation of Aβ (1–16) by either MMP-2 or MMP-9 was not observed even after prolonged incubation times. Especially, all C-terminally truncated Aβ fragments formed by MMP-2 and MMP-9 were highly soluble and did not show fibrillogenic properties or induce cytotoxicity in human cerebral microvascular endothelial or neuronal cells led to the conclusion that these truncated Aβ species are associated with clearance mechanisms rather than being key elements in the fibrillogenesis process. In perspective of AD, beside Aβ, tau protein has also a significant importance. Tau is unusually hyperphosphorylated and aggregated into bundles of filaments [25]. In AD brain this tau pathology is seen as intraneuronal neurofibrillary tangles of Paired Helical Filaments (PHF) sometimes admixed with Straight Filaments (SF). Abnormal hyperphosphorylated aggregation are also seen in dystrophic neurites surrounding the β-amyloid plaque core, and in the neuropil as neuropil threads [26]. Neurofibrillary degeneration of abnormally hyperphosphorylated tau is apparently required for the clinical expression of AD and related tauopathies [27-29]. There are few reports describing the relationship between tau and MMP-2/9. MMP-2 has the capacity to cleave recombinant tau in vitro in a dose-dependent manner, consistent with a physiological function of MMP2 in normal tau proteolysis, although Terni et al proved that MMP-2 does not cleave hyperphosphorylated tau [21]. These observations raise the possibility that accumulation of MMP-2 in neurofibrillary tangles and concomitant loss of proteolytic capacity on tau protein is a response geared to eliminating production of toxic truncated tau species in AD brains. Nubling et al showed a connection between tau and MMP-9 [30]. In this study, the researcher identified MMP-9 as potential tau proteinases. MMP-9 used tau as a substrate, generating specific cleavage patterns [30]. Thus, MMP-2 and MMP- 9 may have therapeutic importance in improvement of AD by the clearance of Aβ and tau.
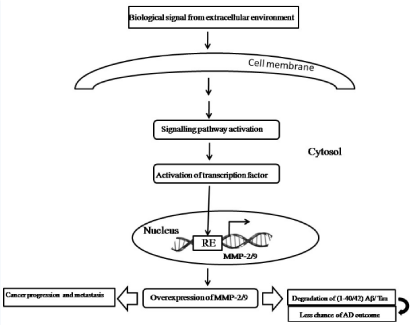
Figure 1: Schematic representation of the role of MMP-2/9 in cancer and AD.
Role of MMP-2 and MMP-9 in Cancer
Over expression of various MMPs has been observed in almost every type of human cancer including brain, breast, lung, ovary and correlates with advanced stage, invasive and metastatic properties and, in general, poor prognosis [31-35]. Among the MMPs, MMP-2 and MMP-9 have been implicated as the most important prognostic factor in cancer microenvironment [36]. A previous study observed the correlation between MMP-2 and breast cancer [37]. MMP-2 was considered along with clinicopathological parameters (tumor size, histological type, nuclear and histological grade, stage, lymph node status, ER, and PR), patients’ survival and Tissue Inhibitor Metallo Proteinase-2 (TIMP-2). MMP-2 immunoreactivity was detected in the cytoplasm in both tumor and tumor stromal cells. MMP-2 reactivity in cancer cells displayed a statistically significant association with tumor size > 2 cm (p = 0.022). Another study indicated that over expression MMP-2 has been noticed in the malignant breast tumors and was increased by paracrine stimulation mediated by soluble factors [38]. A recent study inspected the comparative expression and activity of MMP-9 and its correlation with known pathological parameters such as estrogen receptor, progesterone receptor, and Human Epidermal growth factor Receptor 2 (HER2) in 81 malignant breast tumors and adjacent normal breast tissues and in blood sera of these patients from different clinical TNM stages (ductal carcinoma in situ to T4) of breast cancer [39]. MMP-9 was also highly expressed in node-positive tumors and the preoperative blood serum of patients, but MMP-9 activity was appreciably inhibited in blood serum samples collected after surgery [39]. Another study assessed the prognostic value of MMP-9, by immunoperoxidase staining in a series of 210 breast cancer tissues [40]. MMP-9 staining was observed primarily in cancer cells, and to a lesser degree in surrounding stromal cells but MMP-9 expression was not detected in normal breast tissue [40]. The over expression of MMP-2 and MMP-9 in breast cancer could be used as a prognostic marker to subdivide node negative breast cancer patients in order to determine the optimal treatment modality. Considering other cancer, a recent study examined a correlation between MMP-2 and lung cancer and found that cytoplasmic and stromal expression of MMP-2 has been seen in 39 (54.2%) lung cancer patients [41]. Over expression of MMP-2 was correlated with tumor size, lymph node and distant metastasis. Combined expression of MMP-2 and SDF-1 was significantly correlated with high tumor stage [41]. Several studies also correlated the high expression of MMP-2 and MMP-9 with lung cancer invasion, metastasis and progression [42-46].
MMP-2 and MMP-9 have also been shown to play a crucial role in ovarian cancer progression and metastasis. There are many reports describing the significant role of MMP-2 and MMP-9 in ovarian cancer progression. Hilary et al, using a new organotypic 3D culture of the omentum, showed that MMP-2 was over expressed during ovarian cancer cells adherence, and proteolytically activated in these cells [47]. The activated MMP-2 cleaves the matrix proteins fibronectin, vitronectin and collagen I into smaller fragments. The cleaved ECM fragments then facilitate and accelerate cancer cell adhesion and invasion by binding to their cognate integrin receptors [47]. The study also proved the role of MMP-2 in ovarian cancer by knockdown of MMP-2. In vivo inhibition of MMP-2 before adhesion by using a siRNA or a blocking antibody significantly reduced the number of metastasis and tumor weight in a xenograft mouse model [47]. A recent study also suggested the role of MMP-2 in ovarian cancer progression [48]. This study showed that knockdown of C3G suppressed cell invasion, intravasation and extravasation, and reduced Rap1 activity and secretion of matrix metalloproteinase (MMP)-2 and MMP-9. Thus, C3G-mediated activation of Rap1 could direct the tumor pattern of human ovarian cancer by promoting the secretion of MMP-2 and MMP-9 [48]. Another novel study suggested that Inhibitor of DNA binding/differentiation 1 (Id1) could enhance Endothelial Progenitor Cells (EPC) angiogenesis in ovarian cancer, which is mainly mediated by the PI3K/Akt and NF-κB/MMP-2 signalling pathways [49]. Therefore, Id1 and its downstream effector, probably MMP-2 is potential targets for treatment of ovarian cancer because of its contribution to angiogenesis.
Studies with Colorectal Cancer (CRC), several reports revealed a connection between increased MMP-2 and MMP-9 expression and worse outcome of CRC. Increased level of plasma MMP- 2 expression has been observed in lymph node-positive patients with CRC compared to those without lymph node metastasis [50]. Many studies observed the efficacy of serum MMPs as markers for CRC invasion. One previous study implicated that MMP-2 and -9 protein levels were expressed at significantly higher ratios in the sera of persons with CRC compared to normal controls. This finding had greater diagnostic sensitivity than two other biomarkers currently used in clinical practice, CEA and CA19-9 [51]. In T3-T4 nodenegative CRC, MMP-9 over-expression was associated with poor prognosis. Another study examined the interaction of β1 integrins with MMP-2 in colon cancer cells. These studies demonstrated that MMP-2 was highly expressed in invasive colon cancer cells leading to CRC progression [52].
Conclusions
MMPs, mainly MMP-2 and MMP-9 exert multiple effects in the onset of AD and cancer. Therefore, the clinical utility of these proteins, particularly in plasma or serum, seems to be prospective early diagnostic biomarkers for AD and cancer. Over expression of MMP-2 and MMP-9 seems to be associated with the progression of cancer, however, over expression of these enzymes possibly have preventive effect in AD. Thus, AD and cancer could be comparative with the association of MMP-2 and MMP-9 and may be dependent on the concentration of MMP-2 and MMP-9 level. As a result, although an over expression of MMP-2 and MMP-9 in the brain could lead to the onset of brain tumorigenesis but could have reduced chance to the onset of AD, because an appropriate over expression of MMP-2 and MMP-9 could degrade the most important AD causing factor (1- 40/42) Aβ leading to nontoxic soluble form of Aβ. Ther
References
- Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging. 2006; 27: 190-198.
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993; 101: 64-68.
- Mikuni-Takagaki Y, Cheng YS. Metalloproteinases in endochondral bone formation: appearance of tissue inhibitor-resistant metalloproteinases. Arch Biochem Biophys. 1987; 259: 576-588.
- Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997; 56: 321-339.
- Allsop D, Haga SI, Haga C, Ikeda SI, MannDM, Ishii T. Early senile plaques in Down's syndrome brains show a close relationship with cell bodies of neurons. Neuropathol Appl Neurobiol. 1989; 15: 531-542.
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989; 24: 173-182.
- Wisniewski HM, Wegiel J, Wang KC, Kujawa M, Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989; 16: 535-542.
- Miyakawa T, Shimoji A, Kuramoto R, Higuchi Y. The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Archiv B. 1982; 40: 121-129.
- Kawai M, Kalaria RN, Harik SI, Perry G. The relationship of amyloid plaques to cerebral capillaries in Alzheimer's disease. Am J Pathol. 1990; 137: 1435-1446.
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001; 81: 741-766.
- Millucci L, Ghezzi L, Bernardini G, Santucci A. Conformations and biological activities of amyloid beta peptide 25-35. Curr Protein Pept Sci. 2010; 11: 54-67.
- Palmer JC, Kehoe PG, Love S. Endothelin-converting enzyme-1 in Alzheimer's disease and vascular dementia. Neuropathol Appl Neurobiol. 2010; 36: 487-497.
- Wang XX, Tan MS, Yu JT, Tan L. Matrix metalloproteinases and their multiple roles in Alzheimer's disease. Biomed Res Int. 2014.
- Zhang H, Adwanikar H, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinases and neurotrauma: evolving roles in injury and reparative processes. Neuroscientist. 2010; 16: 156-170.
- Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016; 36: 1481-1507.
- Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, et al. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer's disease. Neurochem Int. 2003; 43: 191-196.
- Romi F, Helgeland G, Gilhus NE. Serum levels of matrix metalloproteinases: implications in clinical neurology. Eur Neurol. 2012; 67: 121-128.
- Tamura Y, Watanabe F, Nakatani T, Yasui K, Fuji M, Komurasaki T, et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J Med Chem. 1998; 41: 640-649.
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007; 101: 566-576.
- Mizoguchi H, Takuma K, Fukuzaki E, Ibi D, Someya E, Akazawa KH, et al. Matrix metalloprotease-9 inhibition improves amyloid beta-mediated cognitive impairment and neurotoxicity in mice. J Pharmacol Exp Ther. 2009; 331: 14-22.
- Terni B, Ferrer I. Abnormal Expression and Distribution of MMP2 at Initial Stages of Alzheimer's Disease-Related Pathology. J Alzheimers Dis. 2015; 46: 461-469.
- Fragkouli A, Tsilibary EC, Tzinia AK. Neuroprotective role of MMP-9 overexpression in the brain of Alzheimer's 5xFAD mice. Neurobiol Dis. 2014; 70: 179-189.
- Horstmann S, Budig L, Gardner H, Koziol J, Deuschle M, Schilling C, et al. Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer’s disease. Int Psychogeriatr. 2010; 22: 966-972.
- Hernandez-Guillamon M, Mawhirt S, Blais S, Montaner J, Neubert TA, Rostagno A, et al. Sequential Amyloid-β Degradation by the Matrix Metalloproteases MMP-2 and MMP-9. J Biol Chem. 2015; 290: 15078-15091.
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986; 83: 4913-4917.
- Braak H, Braak E, Grundke-Iqbal I, Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986; 65: 351-355.
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992; 42: 631-639.
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970; 11: 205-242.
- Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. 1987; 74: 209-225.
- Nubling G, Levin J, Bader B, Israel L, Botzel K, Lorenzl S, et al. Limited cleavage of tau with matrix-metalloproteinase MMP-9, but not MMP-3, enhances tau oligomer formation. Exp Neurol. 2012; 237: 470-476.
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002; 295: 2387-2392.
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat Rev Cancer. 2002; 2: 657-672.
- Lockhart AC, Braun RD, Yu D, Ross JR, Dewhirst MW, Humphrey JS, et al. Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin Cancer Res. 2003; 9: 586-593.
- Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodelling. FASEB J. 1991; 5: 2145-2154.
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997; 89: 1260-1270.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2: 161-174.
- Nakopoulou L, Tsirmpa I, Alexandrou P, Louvrou A, Ampela C, Markaki S, et al. MMP-2 protein in invasive breast cancer and the impact of MMP-2/TIMP-2 phenotype on overall survival. Breast Cancer Res Treat. 2003; 77: 145-155.
- Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, et al. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat. 2002; 72: 69-77.
- Nanda DP, Sil H, Moulik S, Biswas J, Mandal SS, Chatterjee A. Matrix metalloproteinase-9 as a potential tumor marker in breast cancer. J Environ Pathol Toxicol Oncol. 2013; 32: 115-129.
- Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001; 84: 1488-1496.
- Osman NM, Osman WM. SDF-1 and MMP2 cross talk in cancer cells and tumor microenvironment in non-small cell lung cancer. Egyptian Journal of Chest Diseases and Tuberculosis. 2016; 65: 517-525.
- Guo CB, Wang S, Deng C, Zhang DL, Wang FL, Jin XQ. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther. 2007; 11: 183-192.
- Ali-Labib R, Louka ML, Galal IH, Tarek M. Evaluation of matrix metalloproteinase-2 in lung cancer. Proteomics Clin Appl. 2014; 8: 251-257.
- Schveigert D, Cicenas S, Bruzas S, Samalavicius NE, Gudleviciene Z, Didziapetriene J. The value of MMP-9 for breast and non-small cell lung cancer patients' survival. Adv Med Sci. 2013; 58: 73-82.
- Zheng S, Chang Y, Hodges KB, Sun Y, Ma X, Xue Y, et al. Expression of KISS1 and MMP-9 in non-small cell lung cancer and their relations to metastasis and survival. Anticancer Res. 2010; 30: 713-718.
- Hrabec E, Strek M, Nowak D, Hrabec Z. Elevated level of circulating matrix metalloproteinase-9 in patients with lung cancer. Respir Med. 2001; 95: 1-4.
- Kenny HA, Lengyel E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle. 2009; 8: 683-688.
- Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li XM, et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett. 2015; 359: 241-249.
- Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S, et al. Id1 enhances human ovarian cancer endothelial progenitor cell angiogenesis via PI3K/Akt and NF-κB/MMP-2 signaling pathways. J Transl Med. 2013; 11: 132.
- Langenskiold M, Holmdahl L, Falk P, Ivarsson ML. Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Int J Colorectal Dis. 2005; 20: 245-252.
- Dragutinovic VV, Radonjic NV, Petronijevic ND, Tatic SB, Dimitrijevic IB, Radovanovic NS, et al. Matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in preoperative serum as independent prognostic markers in patients with colorectal cancer. Mol Cell Biochem. 2011; 355: 173-178.
- Kryczka J, Stasiak M, Dziki L, Mik M, Dziki A, Cierniewski C. Matrix metalloproteinase-2 cleavage of the beta1 integrin ectodomain facilitates colon cancer cell motility. J Biol Chem. 2012; 287: 36556-36566.