
Special Issue - Neurosurgery
Austin Neurol & Neurosci. 2017; 2(1): 1016.
Atypical Localization of Grossly Calcified Dysembryoplastic Neuroepithelial Tumor (DNT): Case Report
Hakan E¹*, Ahmet HK1,2, Bilal K³, Aziz Y4 and Erol T5
¹Yeni Yuzyil University Gaziosmanpasa Hospital, Department of Neurosurgery, Turkey
²Gelisim University Academy of Health Sciences, Turkey
³Maltepe University, Department of Neurosurgery, Turkey
4Ethica Incirli Hospital, Department of Neurosurgery, Turkey
5Professor of Neurosurgery, Private Consultant, Turkey
*Corresponding author: Hakan Erdogan, Yeni Yuzyil University Gaziosmanpasa Hospital, Department of Neurosurgery, Istanbul, Turkey
Received: April 14, 2017; Accepted: April 27, 2017; Published: May 04, 2017
Abstract
Background: Dysembryoplastic Neuroepithelial Tumors (DNTs) are typically located within supratentorial cortex, often in the temporal lobe. They are frequently present with intractable epilepsy in children and young adults.
Case Report: In our study we report a case of 31-year-old male patient with headache and ataxia during the last six months. His neurological examination revealed no deficit. A right cerebellar mass was detected on his Magnetic Resonance Imaging (MRI). After total resection of the tumor, he was discharged from the hospital on the 10th day of the postoperative period uneventfully. Histological assessment revealed oligodendrocyte-like cells in a microcystic mucinous interstitial background containing “floating” neurons, diagnostic of DNT. In addition, immunohistochemical profile of the neoplasm has supported the diagnosis of DNT.
Conclusion: The present case has unique findings because of the location and gross calcification of the tumor. Only nine cases of cerebellar DNT have been reported in the literature. To our knowledge, both findings in the same tumor have not been reported so far.
Keywords: Dysembryoplastic neuroepithelial tumor; Atypical localization; Gross calcification
Introduction
DNTs are benign tumors of the supratentorial cortex, mostly located in the temporal lobe and they usually present with epileptic seizures in children and young adults [1]. Few studies have reported extracortical locations of DNT, such as cerebellum, brainstem, pericallosal region, septum pellucidum, caudate nucleus, and intraventricular region [2-5]. Secondary germinal layers were thought to be related to these uncommon locations, in accordance with hypothesis of DNT histogenesis [1]. To our knowledge, this is the tenth case of cerebellar DNT, and the first to have massive widespread psammomatous calcification.
Case Report
A 31-year-old man presented with headache and truncal ataxia persisting for the last 6 months. Magnetic Resonance Imaging (MRI) revealed a well countered, heterogenously enhanced mass with 35×45×56 mm dimensions and signal void areas resembling hemorrhage located in the right cerebellar hemisphere which was hypointense on T1-weighted images and hyperintense on T2- weighted images (Figure 1a-d).
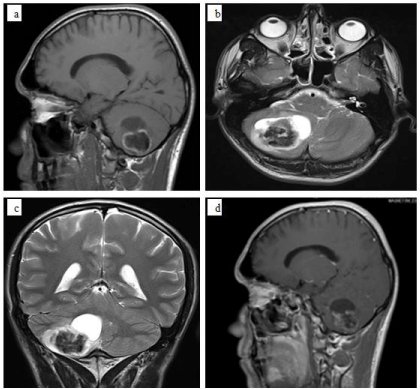
Figure 1: MR images of a 31-year-old patient with cerebellar mass. a) Sagittal
T1-weighted MR image shows a lesion of low signal intensity involving the
right cerebellar hemisphere, b) The lesion is of high signal intensity with
sharp boundaries on an axial T2-weighted MR image, without edema or mass
effect, c) Coronal T2-weighted MR image illustrates the cystic and calcified
components, d) Sagittal T1-weighted MR image shows that the lesion has
heterogeneous enhancement after gadolinium injection.
He underwent to limited craniectomy of posterior fossa and total removal of the tumor was accomplished by piece meal fashion. Peroperative investigation showed superficially located, dark yellowish-brown coloured, partially soft gelatinous and hemorrhagic and mostly calcified mass in the right cerebellar hemisphere (Figure 2). His postoperative period was uneventful and he was discharged from hospital on 10th day postoperatively.
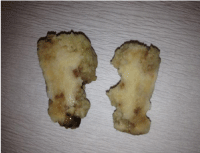
Figure 2: The cut surface of the slices are widely calcified, dark yellowish in
colour and partially hemorrhagic in some areas.
On histological examination, the tumor was composed predominantly of small oligodendrocyte-like cells with round to slightly oval nuclei and perinuclear halos, which were distributed in alveolar and trabecular pattern throughout a mucin-rich background with scattered hypocellular microcysts (Figure 3a). Three fourth of the tumor displayed widespread psammomatous calcification. Nodular architecture was not observed (Figure 3b). A few floating neurons were identified within some parts of the mucinous areas (Figure 3c).
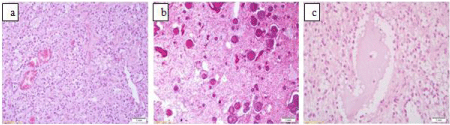
Figure 3: a) Photomicrograph shows oligodendrocyte-like cells in mucinous
matrix (H&E, x 200), b) Widespread psammomatous calcification is stricking
feature(H&E, x 200), c) A few floating neurons are identified within some
parts of the mucinous areas (H&E, x 400).
Immunohistochemical staining showed synaptophysin and neurofilament reactivity within scattered neurons and their processes within the tumor (Figure 4a); however, the small oligodendrocytic cells were nonreactive. Scattered glial fibrillary acidic protein– positive reactive astrocytes were present (Figure 4b). There were no Chromogranin, Pan CK and EMA reactivity and the tumor showed no atypical histological features. The MIB-1 (Ki 67) proliferation index was <1%. The vascularity of the tumor was high and partially tree like structures with goose feet shape and clusters of endothelial proliferations were observed. Chicken-wire patterned microvascular proliferation was also present. The MRI images 6 months after the operation revealed total removal of the tumor (Figure 5 a,b).
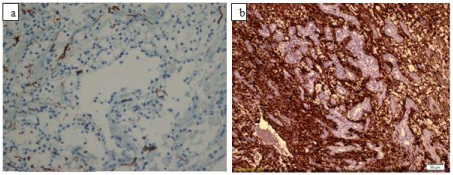
Figure 4: a) In the immunohistochemical staining neurofilament reactivity
within scattered neurons and their processes within the tumor (NF-BSA-x
400), b) Scattered glial fibrillary acidic protein–positive reactive astrocytes
are seen. (GFAP-BSA- x 200).
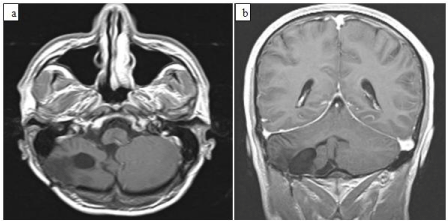
Figure 5: MR images of the paient six months after the operation. a) Axial
T1-weighted MR image shows that the tumor has been removed totally,
b) Coronal T1-weighted MR image after gadolinium injection shows total
removal of the tumor.
Discussion
DNT, first described in 1988 by Daumas-Duport et al [1], constitute a distinct clinicopathological entity among brain tumors. They are rare, benign neoplasms which are included as mixed neuronal-glial tumors in the World Health Organization (WHO) classification [6]. The precise pathogenesis of DNT remains unclear. Cortical dysplasia, disorganized arrangement of neuronal and glial elements without significant cytological atypia are typical for DNTs. They might even be regarded as atypical hamartomas from these reasons [1]. Clinically, DNT is more frequent in children and young adults with intractable epilepsy because of supratentorial and intracortical location. The temporal lobe is the most common site, followed by the frontal, parietal and occipital lobes [1,2]. DNTs present as moderately hypodense lesions on computed tomography and demonstrate decreased signal on T1-weighted images and increased signal on T2-weighted images on MRI with or without enhancement. Cortical location, absence of mass effect and no surrounding edema were suggested as helpful radiological features for diagnosis of DNTs [7].
Cerebellar involvement of DNT is extremely rare and only nine cases were reported in the literature [3,5,8-14]. Eight young adults who had focal lesions presented with similar signs of cerebellar dysfunction and radiological characteristics [3,5,8,10-14]. One of them was reported as complex form variant of DNT, [13] and another one was associated with Lhermitte-Duclos disease [11]. Chiari malformation of the adult type was also reported with one of the cerebellar DNTs [14]. The case of 10-year-old girl with cerebellar DNT showed different clinical and radiological features from others. She was presented with intractable complex partial seizures and behavioral disorder. Her MRI findings revealed multifocal localization, affecting the right temporal lobe, both thalami, right cerebellar hemisphere, and pons [9].
Although the radiological findings were consistent with those of DNT, our preoperative differential diagnoses didn’t include it in the current case. The patient was admitted to hospital with headache and ataxia, presenting atypical clinical manifestations for DNT. The location of the tumor was also unusual for DNT.
Eventually, histopathological evaluation revealed similar features with above-mentioned cerebellar DNTs like oligodendrocyte-like cells, microcysts and floating neurons in a mucinous matrix. Our experience has suggested that DNTs may be more frequent in this location than previously believed. For, diagnosis of a cerebellar DNT can be challenging because DNTs share several features with low grade neoplasms such as central neurocytoma, ependymoma, pilomyxoid astrocytoma, pilocytic astrocytoma, ganglioglioma and glioneuronal malformations, such as hamartomas [1,15]. Histological heterogeneity of cerebellar DNT was also emphasized in a recent study which reported a complex variant of DNT. Vaquero et al have presented a cerebellar tumor with features of pilocytic astrocytoma diagnosed as glioneuronal tumor [13]. Another case report has presented the association of DNT with Lhermitte-Duclos Disease (LDD), which is known as dysplastic cerebellar gangliocytoma. It was suggested that DNT and LDD may represent different points in the spectrum of the same pathology [11]. To distinguish DNTs from oligodendroglioma and neurocytoma is also important so as to avoid unnecessary, potentially harmful aggressive therapy [2,4,9].
Our case is another example of cerebellar DNT, but it is unique because of the gross calcification of the tumor. It is known that DNTs show calcifications very rarely. However, several studies have reported cases presented with calcifications [1,7,16]. Campos et al. have drawn attention to the relationship between calcifications and hemorrhages by stating that calcifications occurred always in the vicinity of hemorrhages. They usually occurred within the subcortical tumor portions predicting that these lesions are complex DNT variants [16]. To the best of our knowledge, grossly calcified DNT has not been previously reported. We present this case to submit one more evidence for unusual behaviour of DNTs. Unusual calcification could be confusing for diagnosis of DNTs, as well as atypical locations.
References
- Daumas-Duport C, Scheitauer BW, Chodkiewicz JP, Laws ER, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988; 23: 545-556.
- Cataltepe O, Marshall P, Smith TW. Dysembryoplastic neuroepithelial tumor located in pericallosal and intraventricular area in a child. Case Report. J Neurosurg Pediatr. 2009; 3: 456-460.
- Fujimoto K, Ohnishi H, Tsujimoto M, Hoshida T, Nakazato Y. Dysembryoplastic neuroepithelial tumor of the cerebellum and brainstem. Case report. J Neurosurg. 2000; 93: 487-489.
- Guesmi H, Houtteville JP, Courthéoux P, Derlon JM, Chapon F. Dysembryoplastic neuroepithelial tumors. Report of 8 cases including two with unusual localization. Neurochirurgie. 1999; 45: 190-200.
- Joh DJ, Leem W, Lim YJ, Kim TS. Dysembryoplastic Neuroepithelial Tumor in the Cerebellum. J Korean Neurosurg Soc. 2002; 32: 173-175.
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993; 3: 255-268.
- Stanescu Cosson R, Varlet P, Beuvon F, Daumas Duport C, Devaux B, Chassoux F, et al. Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up: a study of 53 cases. J Neuroradiol. 2001; 28: 230-240.
- Kuchelmeister K, Demirel T, Schlörer E, Bergmann M, Gullotta F. Dysembryoplastic neuroepithelial tumour of the cerebellum. Acta Neuropathol. 1995; 89: 385-390.
- Leung SY, Gwi E, Ng HK, Fung CF, Yam KY. Dysembryoplastic neuroepithelial tumor. A tumor with small neuronal cells resembling oligodendroglioma. Am J Surg Pathol. 1994; 18: 604-614.
- Litrico S, Desjardins T, Dran G, Michiels JF, Paquis P. Infratentorial Localisation of a Dysembryoplastic Neuroepithelial Tumor. A case report. Neurochirurgie. 2004; 50: 47-52.
- Nair P, Pal L, Jaiswal AK, Behari S. Lhermitte-Duclos disease associated with dysembryoplastic neuroepithelial tumor differentiation with characteristic magnetic resonance appearance of "tiger striping". World Neurosurg. 2011; 75: 699-703.
- Tailor JK, Kim AH, Folkerth RD, Black PM. The development of ring-shaped contrast enhancement in a case of cerebellar dysembryoplastic neuroepithelial tumor: case report. Neurosurgery. 2008; 63: 609-610.
- Vaquero J, Saldaa C, Coca S, Zurita M. Complex form variant of dysembryoplastic neuroepithelial tumor of the cerebellum. Case Rep Pathol. 2012.
- Yasha TC, Mohanty A, Radhesh S, Santosh V, Das S, Shankar SK. Infratentorial Dysembryoplastic Neuroepithelial Tumor (DNT) associated with Arnold-Chiari malformation. Clin Neuropathol. 1998; 17: 305-310.
- Wolf HK, Wellmer J, Muler MB, Wiestler OD, Hufnagel A, Pietsch T. Glioneural malformative lesions and dysembryoplastic neuroepithelial tumors in patient with chronic pharmacoresistant epilepsies. J Neuropathol Exp Neurol. 1995; 54: 245-254.
- Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J, et al. Simple and complex Dysembryoplastic Neuroepithelial Tumors (DNT) variants: clinical profile, MRI, and histopathology. Neuroradiology. 2009; 51: 433-443.