
Research Article
Austin Neurol & Neurosci. 2017; 2(1): 1019.
Comparative Electrophysiological Study of Word Reading in French: Does the P1-N1 Temporal Window Reveal a Neurodevelopmental Anomaly?
Charollais A1,2*, Meyer S2, Ponty C2, Lempereur A2, Stumpf MH1, Berquin P3, Bannier D2, Laudenbach V4, Lalonde R2 and Rebaï M2
¹Reference Center for Language and Learning Disorders and Neuropediatrics, Charles Nicolle University Hospital of Rouen, 1, rue de Germont, F-76031 Cedex, Rouen, France
²CRFDP (Centre de Recherche sur les Fonctionnement et les Dysfonctionnements Psychologiques), University Team EA 4699, University of Rouen, 1 Place Emile Blondel, F-76821 Mont Saint Aignan Cedex, France
³Department of Neuropediatrics, Amiens University Hospital, France
4Rouen Regional Center of Pharmacovigilancy, University Hospital of Rouen, France
*Corresponding author: Charollais Aude, University Charles Nicolle, 1 rue de Germont Rouen 76031, Cedex, France
Received: April 14, 2017; Accepted: June 01, 2017; Published: June 09, 2017
Abstract
Background: Event-Related Potentials (ERPs) permit to study neuronal specialization during reading acquisition. The N170 wave was previously shown to be a surrogate of the fine tuning of reading in adults and adolescents as well.
Aim: We analyzed and described the variations of the N170 wave as a function of French words with visual or phonetical similarities in 12 to 14 yearold dyslexic patients. We tested the validity and modulation of this effect by comparing different populations of normal and dyslexic patients of various severity.
Methods: ERPs were recorded in seventeen dyslexic children with the same method as in normative populations in lexical decision. Stimuli consisted of frequent words chosen on the basis of near or far visemes and morphemes. Dyslexic children were compared to two control (i.e. normal readers) groups, one group of the same age (N=15) and one group of adults (N=17). N170 and P100 waves were analyzed, as well as interactions between both (i.e. P1N1) searched. Psychometric and language tests were also performed. Results were analyzed by ANOVA.
Results: The results of sixteen patients are presented. All sixteen showed significant differences on all psycholinguistic items when compared to the two control groups. All groups (patients and controls) significantly differed from each other for all tests (F’(4;42)=119, 2; p<0.001). However, the heterogeneity of the ERP patterns in the dyslexic group rendered group averaging irrelevant. The N170 wave sometimes overlapped with the P100 wavelength, but was also found to be negative or absent in some patients. In the course of development, N170 variations seem dependent on characteristics of the P100. No correlation was found between the variations of the N170 and clinical measures. Analysis of the P1-N1 temporal course showed a tendency to correlate with reading speed for the entire study population, yet not reaching statistical significance.
Interpretation: N170 variations during development and dyslexic pathologies are associated with P100 variations. The P1-N1 time course could reflect silent reading speed. The P1-N1 temporal course was linked with clinical measures in all three groups, which could reflect neurodevelopmentalyrelated variations of the heterogeneity of the N170 as well as a developmental pathology. Verbal stimuli permit us to test the N170 physiological heterogeneity during development but variations in response to easy tasks show low sensitivity of N170 as a marker of dyslexia.
Keywords: Event-related potentials; Reading; Children; Adolescent; P100; N170; P1-N1 wave
What this Paper Adds-Bullet Points
1. The P1-N1 temporal course appears as a better marker for development and reading automatization than N1 alone.
2. The level of complexity of visual stimuli is of critical importance for a good interpretation of N170 characteristics.
3. The P1-N1 temporal course may be associated with silent reading speed.
4. Our results question the usefulness of exploring the N1 wave alone and add new perspectives on the monitoring of learning abilities in dyslexic children.
Introduction
Reading is a human activity that develops as a consequence of genetic predispositions and a stimulating environment. In electrophysiology, variations in certain Event-Related Potentials (ERPs) in response to reading occur as a function of the paradigm used. Waves may then reflect, allow to decompose or un-mask verbal and visual networks necessary for reading specialization [1]. The N170 wavelength appears as a marker of left-side lateralization as the organism matures and acquires reading competence [2]. Its characteristics depend on the reading expertise of the subject and are sensitive to phonological remediation [3]. Its latency, amplitude, and topography also vary as a function of reading exercises. Importantly, it shows inter-individual variability in every language tested [4].
During grapheme-phoneme conversion when learning the alphabet, the left hemisphere specializes in word recognition. This specialization appears refined when comparing ERPs elicited by words and consonant strings (pseudo-words).
N170 varies as a function of the quantity of lexical storage [5]. However, with augmenting lexical storage, N170 amplitudes increases only for long or more complex words. The amplitude of N170 varies also as a function of the expertise required to encode phono-alphabetic stimuli, which differentiates adult dyslexics from poor readers [6]. The stability of the N170 is highly dependent on methodological factors and the type of stimuli used, which renders its interpretation tricky.
In dyslexic children, neural tuning appears delayed and variations in amplitudes and latencies occur. Indeed, N170 characteristics of normal readers at 8 years of age were found in 11-year old dyslexic children [7]. For the least disabled, a certain form of cognitive economy was possible for frequent words, not different from normal readers but anomalies occurred for decoding processes depending on word length, word frequency and phonological regularity [8]. These results may depend on methodological factors such as the type of task used and the nature of the stimuli, even considering that acquisition and automatization of phono-alphabetic coding is dependent on bimodal invariance as proposed by Hebb’s law [1]. Also, interindividual variability may be the result of the specialization of neural networks subtending visual selective attention required for reading and linked to those of oral language and sound representations [9]. In other words, it may reflect different neurodevelopmental stages reached by the children before they learn to read.
We previously demonstrated that the N170 response to simple visual stimuli occurred as a function of age [10]. In particular, while P100, P200, and P300 components of ERPs were much larger in adolescent than normal adult readers, no difference was observed for N170 and N230 wavelengths, perhaps due to the fact that the underlying neural tuning has already occurred before adolescence or to the relative easiness of the reading task [10]. Here, we describe the N170 variability in dyslexic adolescents (as compared to normal controls) with the same simple approach, aiming to identify specific N170 variations (as compared to P100, P200 and P300).
Methods
The project was approved by the local ethics committee. Experiments were undertaken at the Regional Center for Learning Disorders. The children were recruited among those consulting for learning problems. An informed consent form was signed by at least one parent. Seventeen dyslexic children were evaluated according to the same procedure as reported previously [10]. Fifteen controls were taken from the general population.
Experiments took place in a dedicated room. The lexical decision task was utilized. Tasks measured by EEG occurred in the dark to fixate the subject’s attention on the computer screen. EEG electrodes were connected to an amplifier provided by tMSI (Oldenzaal, the Netherlands). ERPs were recorded with a flexible headset containing 32 electrodes distributed on the scalp according to the international 10-20 system first formulated by Jasper [11]. There were two IBMcompatible computers, one to provide stimuli and the other for data acquisition. The first sent and recorded stimuli via IPRIME software (version 2) and the second collected electrophysiological data or EEG amplified by an amplifier via ASA software (ASA 4.6, ANTNeuro, Enschede, the Netherlands). The impedance of each electrode was verified on ASA software.
For the lexical decision task paradigm, a list of words was presented to the subject as previously reported [10]. The list included words and pseudo-words such as Homo-Phones (HPs) and Written Pseudo-Logatoms (WPLs). HPs are pseudo-words that sound like a real word (e.g. in French « demin » instead of « demain » i.e. « tomorrow »). WPLs are pseudo-words that do not sound like a real word (e.g. « maisson » instead of « maison », i.e. « house »). HPs require an orthographic analysis and WPLs a phonologic analysis. Subjects silently read for 2 min while barring 6 misspelled words. The task consisted of deciding whether a word is real or not among 2-syllable 6-letter words and pseudo-words adapted by us from real words at the following site: www.manulex.org/fr/home.html. Reading speed and reading precision were measured.
Digit Symbol - Coding and Matrix Reasoning subtests of the Wechsler Intelligence Scale for Children-IV were also administered. During Digit-Symbol Coding, subjects copied symbols corresponding to numbers for 2 min, a measure of selective attention and information processing speed. During Matrix Reasoning, a partially filled grid was presented, which had to be completed in a logical manner, a measure of fluid intelligence.
There were 40 blocks of verbal stimuli and a pause every 20 min. Recording and stimulus presentation started at 500 msec, the exposure time was 1 sec, and the maximal allowed time for responding 2 sec.
Statistical analysis
Statistics were run using the Statistica software (Statsoft, Tulsa, CA, US). Comparisons between psycholinguistics (matrices, codes, reading speed, reading precision, leximetry VL ) was performed by ANOVA followed by post hoc analysis by Fischer exact test. A correlation between N170 value and reading speed was searched using Pearson’s test.
Results
The results of sixteen dyslexic patients, 12,6±1 years-old (mean±SD), 8 boys, 8 girls, born at term, presenting with a mixed form of dyslexia, predominantly of the phonological type, at various levels of severity were exploited. Subject 17 was maintained in the analysis for all tests except the lexical decision task due to an incomplete compliance with the correct protocol. The group was compared with 15 normal readers (9 boys, 6 girls) controlled for age (13.4±1 years-old (mean±SD) and 17 normal-reading adults (22.8±2 years-old (mean±SD) 10 men, 7 women). Based on reading speed, subjects were subdivided into a severe form of dyslexia with 3 years of schooling (n=12, 15.6±1.88 years-old), a moderate form with 4 years of schooling (n=4, 31.25±3.66 years-old), a standard form corresponding to their age level with 8 years of schooling (n=10, mean: 41.6, SD:2.06), a precocious form with 8 years of schooling (n=5, mean:60.6, SD:4.85), and an adult form with 11 years of schooling (n=17, mean: 67.31, SD:2.71). ANOVA revealed a global effect (F (4;42)=119,2; p<0.001) and paired comparisons revealed that each group differed from each (p<0,01) (Table 1) other except the precocious versus the adults at borderline level (p<0.051). Dyslexic subjects were at the lower standard level for the two WISC-IV subtests (Digit-Symbol Coding mean: 10 SD:1.5; Matrix Reasoning mean: 10 SD:1.5; For Matrix Reasoning (mean:12.4, SD:1.2) and Digit-Symbol Coding (mean:13, SD:1.44), precocious children were at the 75th percentile, at the adult level, whereas standard children were at the average level. As a whole, dyslexic subjects were inferior to precocious and adult subgroups but similar to standard children, as expected from the definition of the anomaly by the World Health Organization.
TEST
SC
Degré de
MC
F
p
VL
20378,78
4
5094,69
119,244
0,00
HP
3115,95
4
778,99
84,880
0,00
PLE
7022,40
4
1755,60
73,3525
0,00
PL
6691,3
4
1672,8
54,745
0,000000
LUM
21618,6
4
5404,7
45,798
0,000000
Code
135,872
4
33,968
4,5521
0,003817
Matrice
143,021
4
35,755
4,9172
0,002417
COMPARISON 2/2
VL / silent Read speed
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,000004
0,000000
0,000000
0,010565
EP
0,000004
0,051506
0,000000
0,000000
AD
0,000000
0,051506
0,000000
0,000000
DS
0,000000
0,000000
0,000000
0,000169
DM
0,010565
0,000000
0,000000
0,000169
LUM/ Oral Reading speed
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,628517
0,140724
0,000000
0,001362
EP
0,628517
0,096078
0,000000
0,011947
AD
0,140724
0,096078
0,000000
0,000027
DS
0,000000
0,000000
0,000000
0,000443
DM
0,001362
0,011947
0,000027
0,000443
PLE/ Non- word
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,000557
0,000000
0,000000
0,072652
EP
0,000557
0,006590
0,000000
0,000030
AD
0,000000
0,006590
0,000000
0,000000
DS
0,000000
0,000000
0,000000
0,000513
DM
0,072652
0,000030
0,000000
0,000513
HP/ Homophone
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,000008
0,000000
0,000000
0,020599
EP
0,000008
0,424810
0,000000
0,000000
AD
0,000000
0,424810
0,000000
0,000000
DS
0,000000
0,000000
0,000000
0,001408
DM
0,020599
0,000000
0,000000
0,001408
COMPARISON 2/2
PL / reading precision
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,066692
0,000145
0,000000
0,082115
EP
0,066692
0,209133
0,000000
0,003374
AD
0,000145
0,209133
0,000000
0,000015
DS
0,000000
0,000000
0,000000
0,000028
DM
0,082115
0,003374
0,000015
0,000028
CODE
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,199587
0,300180
0,083002
0,205740
EP
0,199587
0,573129
0,008322
0,033544
AD
0,300180
0,573129
0,003464
0,040257
DS
0,083002
0,008322
0,003464
1,000000
DM
0,205740
0,033544
0,040257
1,000000
MATRICE/ Matrices
Reading level
EC
Standard childs
EP
Precocious Childs
AD
Adults
DS severe Dyslexia
DM moderate dyslexia
EC
0,035989
0,011784
0,501212
0,554709
EP
0,035989
0,808200
0,008202
0,026847
AD
0,011784
0,808200
0,000992
0,015277
DS
0,501212
0,008202
0,000992
0,915257
DM
0,554709
0,026847
0,015277
0,915257
Table 1: Overall statistical results and two to two effect of the population for each test.
After eliminating artifacts (-75μv to +75μv) and using grand averaging, we noticed patterns of interest at P7 (left temporo-parietal) and P8 (right temporo-parietal temporo-parietal) electrodes during the lexical decision task for each participant. Irrespective of reading speed, patterns for children contained more positive components than those of adults. The N170 component was absent in severe dyslexia and overlapped the P100 component in some children irrespective of reading speed. The N230 component was always present between 200 and 230 ms. We observed heterogeneity of the N170 component in all groups (Figure 1). No significant difference was detected between N170 patterns in dyslexics and normal readers. Nevertheless, qualitative differences are observable in ERP amplitudes and latencies as well as quantitative differences reflected in inter-individual patterns. We analyzed the positive and negative time course in the 80 to 200 msec range to understand heterogeneity of N170 characteristics (Figure 2). The deviation of the N170 wavelength amid the P100 in all groups performing lexical decisions indicated an association with reading speed in correlation analysis of Pearson test (r=-0,48). The point distribution shows variability in the latency of negativity within the 80-200 ms window in all groups but all the more late as the reading speed (VL) is low. In terms of reading expertise differentiating the groups, the latency distribution before onset of negativity is wider (Figure 3). These results are consistent with the presence of the N170 being superimposed on the P100 in all groups (inter-individual heterogeneity), more so in children. But topographic activity at the 80-200 ms temporal window was more informative in regard to reading activities (Figure 4). The activity of P1-N1 in the 80-200 msec window differentiated normal reading from dyslexic children. In sum, positive and negative activities appeared later and ampler in dyslexic than normal readers for reading simple words in the lexical decision task. The earlier-appearing and less ample positive and negative activities in normal reading children represent a tuning effect.
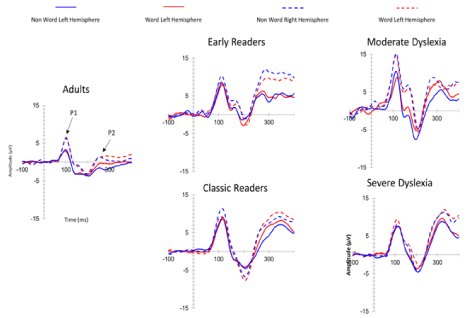
Figure 1: N170 heterogeneity. The N170 component overlapped with the P100 component for child readers reading as quickly as adults. The N170 is not much
different between normal reading children and very severe dyslexic children.
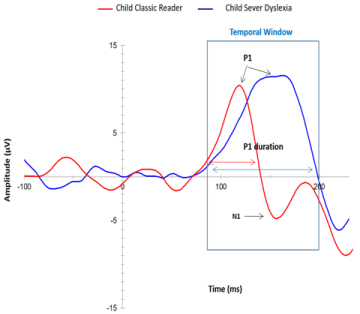
Figure 2: ERPs of normal reading and dyslexic children. It is noted that P1 and N1 components normally found in the 80 to 170 ms window was not found in the
dyslexic brain. Instead, the pattern represents a P1 that overlapped the overall feature of the temporal window to the detriment of the N1.
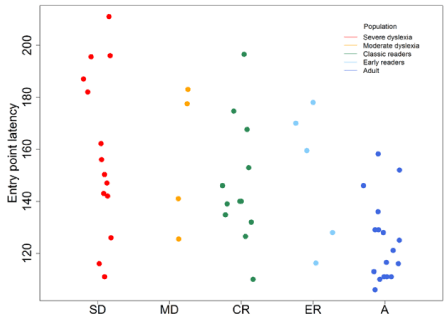
Figure 3: This point distribution shows a variability in the latency of negativity within the 80-200 msec window in all groups. There is an inverse correlation trend
with reading speed. These results are consistent with the presence of the N170 being superimposed on the P100 in all groups (inter-individual heterogeneity), even
more so in children.
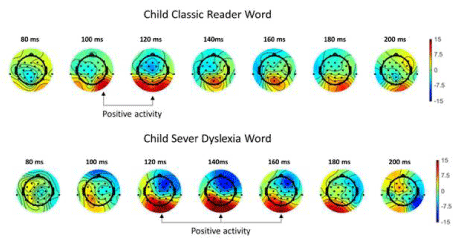
Figure 4: Positive and negative activities appeared later and ampler in dyslexic than normal readers for reading simple words in the lexical decision task. The
earlier-appearing and less ample positive and negative activities in normal reading children represent a tuning effect.
Discussion
In our entire sample, we report an association between reading speed and the P100-N170 time course as well as reading precision on ERPs, all the more so in dyslexic subjects. The N170 component was variable in these dyslexic subjects, as previously described [12]. Variations were masked by the grand averaging method even in normal samples. In non-dyslexic subjects with normal values for reading speed, there was overlap between P100 and N170 wavelengths. Our revealing the P1-N1 interaction may have been due to the fact that stimuli were easy to read even in 7-year-olds. The interaction seems age-related and linked with reading speed and possibly other functional variables. Hasko S showed a diminished area under the curve for the N170 component in developmental dyslexia only for words with false contrasts, interpreted as less efficient encoding relative to normal readers [13]. Our data take into account intersubject N170 variability and agree with the hypothesis that the N170 wavelength varies along with the P100 wavelength and depends on the nature of verbal stimuli.
In addition to these two components, we observed the N230 in all our samples. The N230 wavelength is usually considered to reflect a sub-lexical encoding stage, its presence being explained by the use of our simple stimuli and the schoolwork accomplished by all subjects, including the severely dyslexic group, capable of retrieving common words despite the P1-N1 variability. These results accord with the idea that the P1-N1 relation serves as a dynamic stimulus-dependent variable dependent on maturation. The use of homophones and pseudo-logatoms is relevant in view of previous results presented by Eberhard-Moscicka et al. [14] who emphasized the importance of the use of homophones in lexical storage, utilizing a rapid lexical tract as well as phonologic sub-orthographic abilities, a top-down effect [15]. In this view, word learning is facilitated by sub-orthographic representations but also by a sufficient lexical storage [16].
The P1-N1 interaction likely develops with time, the P100 reflecting selective attention to visually specific stimuli along with a large N170 component, both diminishing in amplitude with age [17]. The N170 is identical for persons reading automatically accessible words and so does not vary as a result of selective attention. However, it varies as a function of type of task used, so that the process of automatization may require both components acting together. This idea explains why the N170 wavelength may be mitigated or overlaps the P100 wavelength in slowly developing readers and why dyslexic subjects have a too ample N170 while reading.
The temporal course showed a tendency to be correlated with reading speed, including homophones and written pseudo-logatoms. The natural evolution of the N170, specializing in automatic reading, always diminishes in amplitude and latency with age. Nevertheless, it seems independent of reading quality but rather dependent on early visual extractions underlying the P100 [18]. Thus, simple word reading in our three groups exposes a neurodevelopmental equilibrium between the P100 and the N170 to acquire automatization. These results should of course be evaluated with a larger sample of subjects as well as younger children with different types of reading problems, since the relatively small number of subjects per group limits our study as well as the small number of studies in which French-speaking subjects read very simple words. P1-N1 coupling may thereby serve as a neurodevelopmental marker of the quality of early reading skills independently of age and pathology. Some reports also indicate the interdependence of P1-N1 coupling during reading exercises [19].
Conclusion
Simple word reading during lexical decisions revealed heterogeneous P1-N1 wavelengths on the EEG in phonetic dyslexic subjects that were associated with reading speed, as were normal young and adult readers. The P1-N1 temporal course provides the best estimate of superimposed neural activity and its dynamic relation with a neurodevelopmental disorder. P1-N1 coupling may explain in part inter-individual variability of the N170 by revealing its construction. This early temporal window permits us to test very quickly early visual extraction from words. Precocious children do not have adult ERPs but instead an N170 overlapping the P100. This original result should permit us to test the hypothesis whether simple words provide a strong contrast to unmask specialization of neural networks underlying the reading process. P1-N1 coupling may be seen as a neurodevelopmental marker of reading skill, since it appears at an early age (7/8 years) and occurs rapidly (<200 msec). The correlation between P1-N1 components reflects a top-down pattern during reading automatization. Analysis of this marker may be extended to other populations such as pre-term subjects to explore neural tendencies during reading acquisition.
References
- Goswami U, Ziegler JC, Richardson U. The effects of spelling consistency on phonological awareness: a comparison of English and German. J Exp Child Psychol. 2005; 92: 345-365.
- Maurer U, Bucher K, Brem S, Benz R, Kranz F, Schulz E, et al. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol Psychiatry. 2009; 66: 341-348.
- Jucla M, Nenert R, Chaix Y, Demonet JF. Remediation effects on N170 and P300 in children with developmental dyslexia. Behav Neurol. 2010; 22: 121- 129.
- Yum YN, Law SP, Su IF, Lau KY, Mo KN. An ERP study of effects of regularity and consistency in delayed naming and lexicality judgment in a logographic writing system. Front Psychol. 2014.
- Perfetti C. Reading Ability: Lexical Quality to Comprehension. Journal Scientific Studies of Reading. 2007; 4: 357-383.
- Mahé G, Bonnefond A, Doignon-Camus N. Is the impaired N170 print tuning specific to developmental dyslexia? A matched reading-level study with poor readers and dyslexics. Brain Lang. 2013; 127: 539-544.
- Maurer U, Brem S, Bucher K, Kranz F, Benz R, Steinhausen HC, et al. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007; 130: 3200-3210.
- Mahé G, Doignon-Camus N, Dufour A, Bonnefond A. Conflict control processing in adults with developmental dyslexia: an event related potentials study. Clin Neurophysiol. 2014; 125: 69-76.
- Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat Rev Neurosci. 2015; 16: 234-244.
- Charollais A, Lempereur A, Simon V, Seraffin C, Lalonde R, Stumpf MH, et al. ERP variabilities as a function of reading maturation. Neurophysiol Clin. 2016; 46: 313-315.
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty system of the International Federation. Electroencephalogr Clin Neurophysiol Suppl. 1999: 52: 3-6.
- Maurer U, Brem S, Bucher K, Brandeis D. Emerging neurophysiological specialization for letter strings. J Cogn Neurosci. 2005; 17: 1532-1552.
- Hasko S, Groth K, Bruder J, Bartling J, Schulte-Körne G. What does the brain of children with developmental dyslexia tell us about reading improvement? ERP evidence from an intervention study. Front Hum Neurosci. 2014; 8: 441.
- Eberhard-Moscicka AK, Jost LB, Raith M, Maurer U. Neurocognitive mechanisms of learning to read: print tuning in beginning readers related to word-reading fluency and semantics but not phonology. Dev Sci. 2015; 18: 106-118.
- Jost LB, Eberhard-Mascicka AK, Frisch C, Dellwo V, Maurer U. Integration of spoken and written words in beginning readers: a topographic ERP study. Brain Topogr. 2014; 27: 786-800.
- Gonzalez-Garrido AA, Gómez-Velázquez FR, Rodríguez-Santillán E. Orthographic recognition in late adolescents: an assessment through eventrelated brain potentials. Clin EEG Neurosci. 2014; 45: 113-121.
- Hasko S, Groth K, Bruder J, Bartling J, Schulte-Körne G. The time course of reading processes in children with and without dyslexia: an ERP study. Front Hum Neurosci. 2013; 7: 570.
- Breznitz Z, Misra M. Speed of processing of the visual-orthographic and auditory-phonological systems in adult dyslexics: the contribution of “asynchrony” to word recognition deficits. Brain Lang. 2003; 85: 486-502.
- Cao F, Rickles B, Vu M, Zhu Z, Chan DH, Harris LN, et al. Early stage visual-orthographic processes predict long-term retention of word form and meaning: a visual encoding training study. J Neurolinguistics. 2013; 26: 440- 461.