
Research Article
Austin Neurol & Neurosci. 2021; 4(1): 1024.
Down-Regulation of Nogo-A and PirB in Ischemic Cortex with Remote Ischemic Preconditioning in Mice
Jiao Y and Wang J*
Departments of Neurology, The Second Affiliated Hospital of Zhengzhou University, China
*Corresponding author: Jinglan Wang, 2 Jingba Road, Zhengzhou, Henan, China
Received: March 01, 2021; Accepted: March 24, 2021; Published: March 31, 2021
Abstract
Objectives: The present study aims to investigate the effect of Limb Remote Ischemic Preconditioning (LRIP) on the expression of Nogo-A and PirB in the cortex of mice with focal cerebral ischemia, and related pathways involving in axonal regeneration and neurological function recovery after cerebral ischemia.
Methods: Adult male C57/BL6 mice were divided into sham-operated (sham), transient Middle Cerebral Artery Occlusion (MCAO), LRIP and anti- PirBAb treatment group. Samples were collected 48h after cerebral ischemia. The histopathologic changes were assessed by 1,3,5-Triphenyl-2H-Tetrazolium Chloride (TTC), and Hematoxylin and Eosin (HE) staining and TUNEL method. The expression of Nogo-A and PirB were determined by immunofluorescence, RT-PCR and Western blot respectively.
Results: TTC staining showed that LRIP treatment reduced the infarct size of mice and anti-PirBAb treatment further decline the infarct size, which was accompanied with the decline of neurological deficit score and reduction of neuronal damage. LRIP treatment also reduced the TUNEL positive cells induced by MCAO and anti-PirBAb treatment further strengthened the effect of LRIP. Except sham group, the expressions of Nogo-A and PirB in other three groups all increased with varying degrees, among which MCAO group was the highest, LRIP group was the second and the anti-PirBAb group was the lowest. The expressions of growth associated protein 43 (GAP43) showed opposite tendency.
Conclusions: LRIP plays beneficial influence on cerebral ischemia. LRIP and PirB inhibition combination has a better protective effect on nervous system after cerebral ischemia in mice.
Keywords: Limb Remote Ischemic Preconditioning; Cerebral ischemia; Nogo-A; PirB; Neuroprotection
Abbreviations
CNS: Central Nervous System; HE: Hematoxylin and Eosin; LRIP: Limb Remote Ischemic Preconditioning; MCA: Middle Cerebral Artery; MCAO: Middle Cerebral Artery Occlusion; mNSS: Modified Neurological Severity Score; NgR: Nogo Receptor; Nogo: Neurite Outgrowth Inhibitor; rCBF: Regional Cortical Cerebral Blood Flow; TTC: 2,3,5-Triphenyl-Tetrazolium Chloride.
Introduction
With the improvement of human living standard and the extension of average life expectancy, cerebrovascular disease has been becoming a severe threat to human life and health. As the most common type of cerebrovascular disease, ischemic cerebrovascular disease accounts for about 70% of all cases [1]. It has been reported that Limb Remote Ischemic Preconditioning (LRIP) could induce ischemic tolerance and reduce the nerve injury caused by ischemia [2]. In recent years, LRIP was considered as a feasible therapeutic strategy for stroke. Some relevant experiments were carried out on rat, which proved that LRIP could provide a protective effect on stroke [3-5].
Neurite Outgrowth Inhibitor-A (Nogo-A) is an important nerve growth suppressor that plays an inhibitory role in regeneration of neurons. Previous study demonstrated that the degree of functional damage after cerebral ischemia was closely related to the difficulty of neuron regeneration, which suggested Nogo-A might take part in the regulation of functional injury after cerebral ischemia. Grandpre et al. [6] indicated that inhibition of Nogo Receptors (NgR) could promote regeneration of damaged neurons. But the results of Zheng et al. [7] pointed out, that the inhibitory effect of myelin inhibitor in NgR knockout mice and wild mice was no significant difference. Therefore, maybe there is a second receptor that mediates the inhibition of myelin inhibitor exists. Earlier research found that Paired Immunoglobulin-Like Receptor B (PirB) was expressed in the central nervous system [8]. They discovered that the mRNA and protein expressions of PirB could be detected in the whole brain tissue of adult mice, and PirB protein was mainly expressed in the cerebral cortex, hippocampus, cerebellar neurons, axons, and synapses. Moreover, as the same with NgR, PirB could combine with Myelin-associated glycoprotein, Nogo-A and oligodendrocyte myelin glycoprotein to inhibit nerve regeneration [9]. Cerebral cortex is the most sensitive part of the brain to ischemia, and its structural integrity is the basis of cognitive function. Therefore, it is speculated that the up-regulated expression of Nogo-A in the cerebral cortex may be a reason for cognitive function decline after cerebral ischemia injury, which leads to decreased neuroplasticity and reduced the ability of regeneration and repair [10-12]. In this study, we investigated the expression of Nogo-A and PirB in the cortex of mice with cerebral ischemia after LRIP, and the related pathways involving in axonal regeneration and neurological function recovery after cerebral ischemia.
Materials and Methods
Experimental animals
A total of 120 adults C57/BL6 mice (male, weighing 20-25 grams) were obtained from the central animal facility of Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). All mice were reared at a temperature of 23±2°C in a 50±5% humiditycontrolled room with a 12-hour light-dark cycle with a standard diet. All the experiments were performed strictly according to ’Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health, USA20. After fed in our laboratory for one week, the mice were randomly divided into following groups using a lottery-drawing box: 1) Sham-operated group (sham); 2) Middle Cerebral Artery Occlusion (MCAO); 3) LRIP + MCAO (LRIP); 4) LRIP + MCAO + anti PirB antibody (anti-PirBAb) treatment group. All outcome evaluations were carried out by investigators blinded to groups. The research protocols were approved by the Institutional Animal Ethics Committee of Zhengzhou University (Zhengzhou, China).
Limb remote ischemic preconditioning
Noninvasive LRIP was performed as described previously [2,13,14]. LRIP was conducted on both hindlimbs of mice anesthetized with 1-3% isoflurane by non-invasive occlusion of hind limb blood flow with a gauge bandage, in contrast to an invasive, direct femoral artery occlusion. The two hind limbs were simultaneously tied with a bandage to occlude blood circulation for 10min and then released for 10min to allow for reperfusion. The occlusion/reperfusion cycle was repeated three times.
MCAO model
MCAO was performed as described previously [15]. Briefly, mice were anesthetized with 1.5~2% isoflurane. Then, the mice were placed with a supine position, fur was sterilized with 70% ethanol. With the aid of a dissecting microscope, a skin incision was made on the right side of the head from the anterior of the ear toward the corner of the eye horizontally and from the corner of the eye vertically 5mm. Using a fine battery-powered drill (Dremel), a small hole was made 2mm in diameter on the skull bone to remove dura and expose the MCA. The MCA was then cauterized using an electronic coagulator (Codman & Shurtleff), and the incised muscle layer and skin were sutured. In the sham operation group, the same craniotomy was performed, but the middle cerebral artery and its branches were not electro coagulated. In the LRIP group, the mice underwent distal ischemic preconditioning before MCAO surgery. In anti-PirBAb group, mice were subjected to the same protocol as LRIP group, however, after 2h of ischemia, mice were given an intracranial injection of anti-PirB antibody.
In the experiment, Regional Cortical Cerebral Blood Flow (rCBF) was monitored by laser Doppler flowmetry (Periflux System 6000, Olympus, Germany) in the ipsilateral cortex and rectal temperature was monitored and maintained at 37oC (90303B, Spacelabs Healthcare, Snoqualmie, WA, USA). The rCBF values were obtained 5min before and after MCAO, and 5min after the reperfusion of CIP. Mice in the MCAO group (after recovery from anesthesia) that had rCBF value above 40% of baseline were excluded from the study.
To minimize pain and infections after operation, mice were given daily subcutaneous injections of Rimadyl® (2.5mg/kg, Pfizer) and Baytril® (5mg/kg, Bayer) for consecutive 2 days after surgery.
Infarct size measurement
2,3,5-Triphenyl-2H-Tetrazolium Chloride (TTC) staining was used for measuring the infarct size after 48h of reperfusion when the mice were euthanized. Infarct volume was determined using ImageJ analysis by an observer blinded to the experimental group assignment. We evaluated the infarction area ratio using the following method: infarction area ratio = (white infarct area × thickness)/(whole slice area × thickness) ×100%. The detailed protocol has been described previously [16].
Behavioral testing
Modified Neurological Severity Score (mNSS) were examined by 2 investigators who were blinded to the experimental groups to assess sensorimotor as described in a previous study [17]. Neurological function is graded on a scale of 0 to 18 (normal score, 0; maximal deficit score, 18), and the higher score represents the greater severity of the injury.
Hematoxylin and Eosin (HE) staining
The areas of brain injury were evaluated by HE staining. Briefly, brain tissues were cut into 5μm thick paraffin-embedded sections, and then the sections were dried at 50°C for at least 30min. Routine hematoxylin and eosin staining was used to evaluate brain injury areas. The sections were processed as described previously [18].
Tunel staining
Terminal deoxynucleotidyl Transferase-mediated dUTP Nick End Labeling (TUNEL) was used to identify dying cells with damaged DNA in each group according to the instructions of TUNEL kit (Roche, USA). Positively stained cells were counted using ImageJ software.
Immunofluorescence
The mice in each group (n=5) were sacrificed under anesthesia with 1.5~2% isoflurane. The expressions of Nogo-A and PirB in the cerebral ischemia of mice were also detected by immunofluorescence method. The dilution ratio of anti-Nogo-A antibody was 1:1000 (Santa Cruz, USA) and the dilution ratio of anti-PirB antibody was 1:200 (Santa Cruz, USA). Results were observed and obtained by inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
The mice in each group (n=5) were sacrificed under anesthesia with 1.5~2% isoflurane. The cerebral cortex of mice was collected on ice and stored at -80°C immediately after the mice were euthanized. Samples were homogenized in RIPA lysis buffer (Catalog No. R0020, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and centrifuged at 12000 x g for 15min at 4°C. Then, the total proteins in the supernatant were collected and the concentration of protein was determined using bicinchoninic acid protein assay kit (Catalog No. BCA02, Beijing Dingguo Changsheng Biotechnology Co., Ltd., BeiJing, China). Followed by electrophoresing and transferring, the proteins in Nitrocellulose (NC) membrane (Millipore, USA) were incubated with anti-Nogo-A antibody (1:1000, ab62024, Abcam, USA), anti-PirBantibody (1:10000, ab170909, Abcam, USA), antigrowth associated protein 43 (GAP43) antibody (1:1000, ab75810, Abcam, USA) and anti-β-actin antibody (1:5000, ab8227, Abcam, USA) respectively overnight at 4oC. After washing with TBS-T, the membrane was incubated with secondary antibodies for 1h at room temperature. The immunoreactivity was visualized with Super ECL Plus detection reagent (PE0010, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) using Bio-Rad Gel Doc XR+ (Hercules, USA). Band intensity was analyzed using ImageJ software (Olympus, Germany). The ratios of gray scale values of target proteins to internal control (β-actin) were used to measure the relative amount of proteins.
Quantitative RT-PCR analysis
The mRNA expressions of Nogo-A and PirB in the mice cortex were evaluated by RT-PCR. Briefly, the mice (n=5) were sacrificed to obtain right cerebral cortex from each group at the time point. Total RNA was extracted with the Trigol reagent (DH353-2, Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China). Primed RNA (1μg) was reverse transcribed with PrimeScriptTM RT Master Mix (Code No. RR036Q, Takara, Otsu, Shiga, Japan) according to the manufacturer’s instructions. The reverse-transcription condition was 37°C for 15min followed by a final termination step at 85°C for 5s. cDNA was amplified by PCR using 7500 Real-Time PCR System (Applied Biosystems, CA, USA) with the following conditions: initial denaturation at 95°C for 30s (1 cycle), denaturation at 95oC for 5s, annealing for 60°C for 35s (40 cycles), dissociation stage. The relative mNRA expressions of Nogo-A and PirB were calculated by the grayscale ratios of DNA bands of both Nogo-A and PirB in relation to the internal control (β-actin). Primers sequences were as follows: Nogo-A–forward: 5’ - A A G G T G A G T C A C G C C A A A C T G - 3’, reverse: 5’ - C T T T C G G T T G C T G A G G T A - 3’; PirB–forward: 5’ - G A C T T A T G C C C A G G T G A A A C C - 3’, reverse: 5’ - A G A T T C G G C A G C C T G A T T G T T - 3’; GAP43- forward: 5’ - A C C T A A G G A A A G T G C C C G A C - 3’, reverse: 5’ - G C A T C G G T A G T A G C A G A G C C -3’; β-actin–forward: 5’ - C A G T G C C A G C C T C G T C T C A T - 3’, reverse: 5’ - A G G G G C C A T C C A C A G T C T T C - 3’.
Statistics analysis
Experimental data were presented as Mean ± Standard Deviation. SPSS Statistics for Windows version 18.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. Significant differences were analyzed using one-way ANOVA. Statistical significance was accepted for P values of less than 0.05.
Results
Infarct size in 4 groups
We first performed TTC staining to determine the area of cerebral infarction. The results of TTC staining showed that the infarct size in MCAO group was significantly larger than that in sham operation group (P<0.001). MCAO group had the largest infarct size. LRIP treatment could reduce the size of infarct induced by MCAO (P<0.001) and anti-PirBAb treatment further decline the infarct size (P<0.05, Figure 1A).
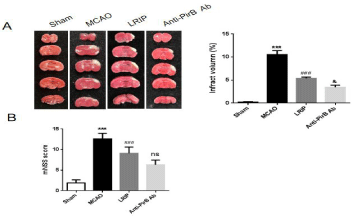
Figure 1: (A) Representative images and infarct volume of mice in the sham,
MCAO, LRIP and anti-PirBAb group after 2,3,5-triphenyl-2H-tetrazolium
chloride (TTC) staining (n=5 per group). (B) Neurological score.
***P<0.001, Sham versus MCAO; ###P<0.001, MCAO versus LRIP; &P<0.05 or
no significant (ns), LRIP versus Anti-PirB Ab.
Then, the neurological deficit score was used to assess the degree of brain damage after 48h of MCAO. Result displayed that the mice in the sham operation group showed no neurological deficit, and the mice in other three groups all showed different degrees of neurological deficit. Compared to MCAO group, the neurological deficit score was reduced in the LRIP group (P<0.001), and was significantly different from that in MCAO group. In addition, there was a lower neurological deficit score in anti-PirBAb group in relative to the LRIP group (No significant, ns, Figure 1B).
These findings confirm that LRIP triggers structural and functional protection against stroke in mice and on this basis, the protection effect of blocking PirB is more obvious.
Histopathologic changes
After 48h of focal cerebral infarction, mice brains were collected for detecting histopathological damage. Figure 2 showed the representative samples of H&E staining from brain tissues of the sham control and modeling groups. In the sham group, the cortical neurons were arranged in a regular pattern, with larger and darker nuclei, oval shape and located in the center of the cells. No damage or edema was observed on nerve cells in sham group. In the MCAO group, many necrotic cells and nuclear contraction and structural disorder appeared in the cerebral cortex neurons. Compared to MCAO group, the number of cortical neurons was increased and the shape was more regular in LRIP group and anti-PirBAb group. The shape of neurons in anti-PirBAb group was closest to that of in the sham group.
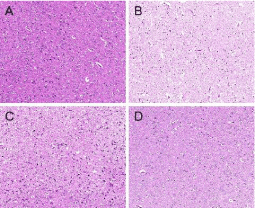
Figure 2: Infarcted brain tissue of Hematoxylin-Eosin Staining (HE) in the
sham (A), MCAO (B), LRIP (C) and anti-PirBAb (D) group in mice (200X, n=5
per group). In the sham group, the cortical neurons were arranged in a regular
pattern, with larger and darker nuclei, oval shape and located in the center of
the cells. No damage or edema was observed on nerve cells in sham group.
In the MCAO group, a large number of necrotic cells and nuclear contraction
and structural disorder appeared in the cerebral cortex neurons. Compared
to MCAO group, the number of cortical neurons was increased and the shape
was more regular in LRIP group and anti-PirBAb group. The shape of neurons
in anti-PirBAb group was closest to that of in the sham group.
TUNEL staining was used to assess the cell death including necrosis and apoptosis after brain ischemia. The results showed that there were few TUNEL positive cells in the sham operation group and the TUNEL positive cells in the other three groups were increased with different degrees. The maximum number of TUNEL positive cells was presented in the MCAO group (Figure 3). Compared to the MCAO group, percentages of TUNEL positive cells in the LRIP group and the anti-PirBAb group were significantly reduced (P <0.001). In addition, compared to the LRIP group, the number of TUNEL positive cells in the anti-PirBAb group was further decreased (P <0.01).
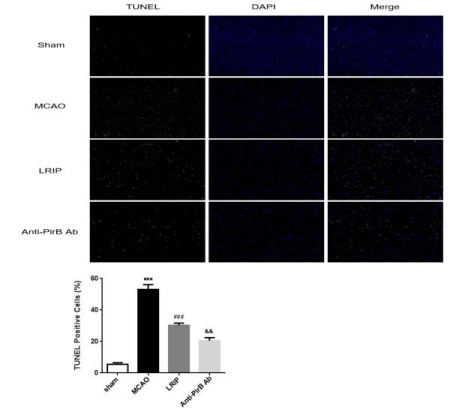
Figure 3: TUNEL and percentage of TUNEL positive cells in the brain issue in
sham, MCAO, LRIP and anti-PirBAb group (200X, n=5 per group).
***P<0.001, Sham versus MCAO; ###P<0.001, MCAO versus LRIP; &&P<0.01,
LRIP versus Anti-PirB Ab.
Expression and distribution of Nogo-A and PirB
To determine the expression and distribution of Nogo-A and PirB, we performed immunofluorescence using paraffin embedded sections from each group. A few Nogo-A positive immune-reactive cells were detected in the sham group, and the expression of Nogo-A positive cells increased in the other three groups, among which MCAO group was the highest, LRIP group was the second and the anti-PirBAb group was the lowest (Figure 4). Positive immunoreactive cells were mainly distributed in the core of the ischemic cortex. The trend of PirB expression was similar with that of Nogo-A (Figure 5).
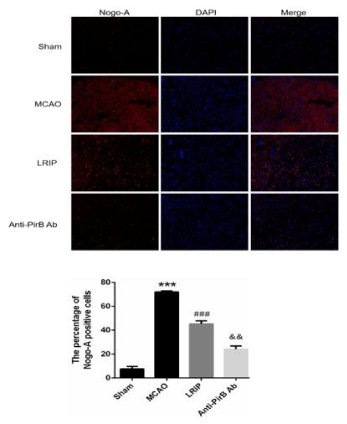
Figure 4: The expression of Nogo-A proteins was detected by
immunofluorescence in each group (200X, n=5 per group).
***P<0.001, Sham versus MCAO; ###P<0.05, MCAO versus LRIP; &&P<0.01,
LRIP versus Anti-PirB Ab.
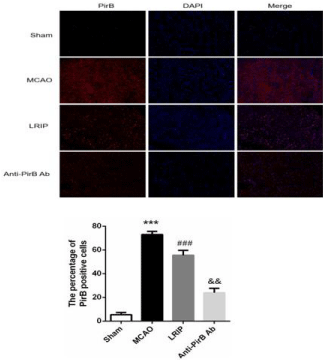
Figure 5: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Nogo-A and PirB mRNA and protein expression
To quantify Nogo-A and PirB expressions in each group, we measured the mRNA and protein expressions of Nogo-A and PirB using RT-PCR and Western blot analysis. RT-PCR results showed that the mRNA expressions of Nogo-A and PirB in MCAO group were higher than that in the sham group (Figure 6, P<0.001). LRIP treatment reduced the mRNA expressions of Nogo-A and PirB induced by MCAO (P<0.001). Besides, the mRNA expressions of Nogo-A and PirB in anti-PirBAb group were further declined compared to LRIP group (P<0.001). However, the mRNA expression of GAP43 showed opposite tendency (Figure 6). Similar results were found concerning the protein expressions of Nogo-A, PirB and GAP43 (Figure 7).
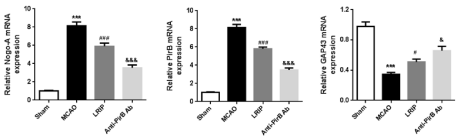
Figure 6: The mRNA levels of Nogo-A, PirB and GAP43 were assessed by real-time RT-PCR (n=5 per group). ***P<0.001, Sham versus MCAO; #P<0.05 or
###P<0.001, MCAO versus LRIP; &P<0.05 or &&&P<0.001, LRIP versus Anti-PirB Ab.
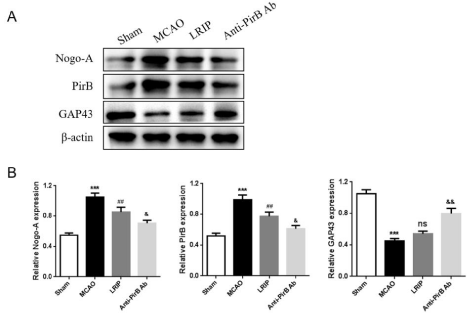
Figure 7: (A) The protein expressions of Nogo-A, PirB and GAP43 of each group were determined by Western blotting. (B) Quantitative analysis of WB (n=5 per
group).
***P<0.001, Sham versus MCAO; ##P<0.01 or ns, MCAO versus LRIP; &P<0.05, &&P<0.01, LRIP versus Anti-PirB Ab.
Discussion
Stroke, resulting from limited blood flow to the brain, is classified as ischemic, due to lack of blood flow, or hemorrhagic, due to bleeding, and the majority of stroke is ischemic (87%) [19]. Due to the disorder of blood supply in the brain, the cerebral tissue in the blood vessel supply area has ischemia and hypoxia, and neuron death eventually occurs. Previous literature proved that after the Central Nervous System (CNS) injury, it is difficult to recover by promoting axon regeneration and neuron contacts [20]. The difficulties in repairing and regenerating nerve cells and conduction pathways are the main cause of the residual nerve dysfunction.
LRIP is an endogenous protective mechanism through shortterm sub-lethal ischemia in a remote organ to protect the major organ from further severe ischemia. Previous study demonstrated that in an in vivo model of myocardial infarction, the short periods of limb ischemia of LRIP reduced experimental infarct size by 50% [21]. Induction of the LRIP by transient limb ischemia appears to be an attractive, safe, and practical approach in clinical practice. Although beneficial effects of the remote ischemic preconditioning have been demonstrated, both in animals and in humans, its effect on neuronal death remains controversial [22-25]. A recent study in rats demonstrated that delayed or second window LRIP induced by transient lower limb ischemia significantly reduced cerebral infarction size after combined focal and global cerebral ischemia [26]. In this study, LRIP group observed a decrease in cerebral infarction size at 48h after cerebral ischemia, as well as the reduced neurological deficit score, the brain damage and death cells, which further confirm the protective effect of LRIP on stroke.
The difficulty in nerve repair after mature CNS injury is mainly due to the Myelin-Associated Inhibitors (MAIs) present in the central microenvironment inhibit axonal regeneration [27], and Nogo-A is the most potent inhibitor. The importance of Nogo-A in limiting axonal growth in vivo has been demonstrated. In this study, we established the mouse model of MCAO to investigate the changes of expression in Nogo-A and PirB after relevant treatment. The findings presented that Nogo-A and PirB have the uniformity in the results for immunoreactivity, protein, and gene expression patterns of across all the groups. Tews et al. [28] confirmed that Nogo-A knockout mice showed abnormal memory damage behavior in the process of development, which explained that Nogo-A played regulatory role in pubertal neurons and synaptic plasticity and stability. In our experiment, the mice in Sham group rats had a small Nogo-A expression, that may be used to maintain the stability of the neural circuits, maintain normal rats learning and memory and so on. Nogo-A expression in cerebral hemorrhage of the rats had the potential to increase, and cerebral hemorrhage rats appeared obvious nerve dysfunction. Prompting Nogo A in cerebral hemorrhage of neurons and axons while repairing the negative regulation, which hinders the recovery of neural function. In 2006, Syken et al. [8] confirmed PirB expression in the CNS, which expressed during the entire development process to adjust the neurons stable guide synaptic plasticity. Wang et al. [29] demonstrated that the expression levels of Nogo-A and its receptors are up-regulated after ischemia, indicating their potential roles in the pathological progression of ischemia. Another study indicated that PirB was a significant element in the pathological process of cerebral ischemia [30]. Moreover, earlier study proved that inhibition of Nogo-A activity by neutralizing protein after stroke markedly promotes regeneration of the corticospinal tract and restoration of neurological function [31]. PirB was discovered to inhibit the neuron regeneration in newborn rat hypoxic-ischemic brain injury model [1]. In our study, we revealed that LRIP and anti-PirB antibody treatment obviously decreased the cerebral infarct size in the mice model of focal ischemia. It suggested that LRIP and PirB blocking combination has a better protective effect on the cerebral ischemia.
Besides, we also found that LRIP decreased the mRNA and protein expressions of Nogo-A and PirB in the cerebral cortex. A previous study reported that PirB exerts negative regulation on axonal regeneration in the visual cortex [8]. Similar results were also observed in our in vivo cerebral ischemia model. These results suggested that Nogo-A and PirB also participated in the process of LRIP. As a membrane-anchored neuronal growth associated protein, GAP43 joins in the regulation of novel neuronal connection formation and synaptic remodeling after damage [32]. Herein, we discovered that the GAP43 expression was decreased after MCAO. LRIP treatment up-regulated the GAP43 expression in the cerebral cortex and anti- PriBAb antibody treatment further raised the GAP43. These results implied that GAP43 exerted opposite influence in MCAO, compared to Nogo-A and PirB, which may form a complex regulatory network. More study is needed in future to further exploring the effect of GAP43 in LRIP.
Taken together, this research further confirmed the beneficial effect of LRIP on cerebral ischemia. Most importantly, we proposed that combination inhibition both LRIP and PirB has a better protective effect on nervous system after cerebral ischemia.
Author Contributions
Yiming Jiao wrote the first manuscript and did the experiments; Jinlan Wang drew the images and edited the manuscript; they both supervised the project.
Acknowledgements
We are grateful to the numerous individuals who participated in this study.
Funding
The article was supported by Jonit Project of Henan Province Medical Science and Technology Research Plan (grant no: LHGJ20190313).
References
- Weih M, Prass K, Ruscher G, et al. Ischemia tolerance; model for research, hope for clinical practice? Der Nervenarzt. 2001; 72: 255-260.
- Du X, Yang C, Liu S, et al. Hypoxia-Inducible factor 1a and 2a have beneficial effects in remote ischemic preconditioning against stroke by modulating inflammatory responses in aged rats. Frontiers in Aging Neuroscience. 2020; 12: 54.
- Wei C, Ren X, Chen Z, et al. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLOS ONE. 2012; 7: e30892.
- Zong-Jian L, Xiao-Rong C, Yuan-Yuan L, et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is sssociated with significant alterations in peripheral immune responses. CNS Neuroscience & Therapeutics. 2015; 24: 34-38.
- Yang C, Liu X, Du M, et al. Hypoxia inducible factor 1alpha plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J Am Heart Assoc. 2018; 7: e007589.
- Grandpré T, S Li and SM Strittmatter. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002; 417: 547-551.
- Zheng J, Atwal C, Ho L, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003; 102: 1205-1210.
- Syken T, GrandPre P, Kanold C, et al. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006; 313: 1795-1800.
- KJ Atwal, J Pinkston, Gosse J, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008; 322: 967-970.
- Lee J-K. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. Journal of Neuroscience the Official Journal of the Society for Neuroscience. 2004; 24: 6209-6217.
- Yin Y, Z Chen and Z Du. Cognitive rehabilitation and neuroplasticity after stroke. Continuing Medical Education. 2007; 20: 471-474.
- Zhu W. Cognitive function and neural plasticity the basic mechanism of improving cognitive function of ginsenoside rg1 due to its regulation for neural plasticity. Herald of Medicine. 2007: 11-17.
- Hu S, Dong H. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Research. 2012; 1459: 81-90.
- Hu Y, Lu Y, Zhang Y, et al. Remote ischemic preconditioning improves spatial learning and memory ability after focal cerebral ischemia-reperfusion in rats. Perfusion. 2013; 28: 546-551.
- Nakase G, Sohl M, Theis K, et al. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol. 2004; 164: 2067-2075.
- Liu X, R Hu, L Pei, et al. Regulatory T cell is critical for interleukin-33-mediated neuroprotection against stroke. Exp Neurol. 2020; 328: 113233.
- Bai YY, X Gao, YC Wang, et al. Image-guided pro-angiogenic therapy in diabetic stroke mouse models using a multi-modal nanoprobe. Theranostics. 2014; 4: 787-797.
- Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. The Journal of neuroscience. 2006; 26: 10281-10291.
- Donnan GA, Fisher M, et al. Stroke. Lancet. 2008; 371: 1612-1623.
- Hermann D. Promoting brain remodeling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012; 11: 369-380.
- Kharbanda RK, UM Mortensen, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002; 106: 2881-2883.
- Heurteaux C, I Lauritzen, C Widmann, et al. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad. 1995; 92: 4666-4670.
- Matsuyama K, Y Chiba, A Ihaya, et al. Effect of spinal cord preconditioning on paraplegia during cross-clamping of the thoracic aorta. Ann Thorac Surg. 1997; 63: 1315-1320.
- Sakurai M, T Hayashi, K Abe, et al. Enhancement of heat shock protein expression after transient ischemia in the preconditioned spinal cord of rabbits. J Vasc Surg. 1998; 27: 720-725.
- Ondrejcak T. Ischemic preconditioning does not improve neurological recovery after spinal cord compression injury in the rat. Brain Res. 2004; 995: 267-273.
- Ren C, X Gao. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008; 151: 1099-1103.
- Wang KC, V Koprivica, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002; 417: 941-944.
- Tews B, K Schonig, ME Arzt, et al. Synthetic microRNA-mediated downregulation of Nogo-A in transgenic rats reveals its role as regulator of synaptic plasticity and cognitive function. Proc Natl Acad. 2013; 110: 6583- 6588.
- Wang F, Z Liang, Q Hou, et al. Nogo-A is involved in secondary axonal degeneration of thalamus in hypertensive rats with focal cortical infarction. Neurosci Lett. 2007; 417: 255-260.
- Wang J, Y Zhang, J Xia, et al. Neuronal PirB upregulated in berebral ischemia acts as an attractive theranostic target for ischemic stroke. J Am Heart Assoc. 2018; 7: e007197.
- Tsai S-Y, CM Papadopoulos, et al. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke. 2011; 42: 186-190.
- Hung C. Astrocytic GAP43 induced by the TLR4/NF-κB/STAT3 axis attenuates astrogliosis-mediated microglial activation and neurotoxicity. Stroke. 2016; 36: 2027-2043.