
Case Report
Austin Neurol & Neurosci. 2022; 5(1): 1027.
Guillain-Barré Syndrome Following Anterior Cervical Spine Surgery and COVID-19 Vaccination: Literature Review with a Case Report
Cavagnaro MJ¹*, Orenday-Barraza JM¹, Georges J² and Baaj AA¹
1Department of Neurosurgery, University of Arizona College of Medicine, AZ, USA
2Department of Neurosurgery, Philadelphia College of Osteopathic Medicine, Philadelphia, Pennsylvania, USA
*Corresponding author: María José Cavagnaro, Department of Neurosurgery, University of Arizona College of Medicine, 475 N 5th St, Phoenix, AZ, USA
Received: February 22, 2022; Accepted: March 18, 2022; Published: March 25, 2022
Abstract
Coronavirus Disease 2019 became a pandemic in March 2020 and vaccines were developed as the most efficient and effective means to control the disease. However, during the early vaccination period, side effects were reported. The following presents a case of GBS with a temporal relationship to COVID-19 vaccination occurring in a patient who recently underwent spinal surgery. In addition, a literature review was performed to draw attention to this neurological complication in association with COVID-19 vaccination and/or spinal surgery.
Keywords: ACDF; Cervical myelopathy; COVID-19; Guillain barre syndrome; Vaccine
Introduction
Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), became a pandemic in March 2020 [1], causing significant economic and health concerns worldwide. Vaccines were developed as the most efficient and effective means to control the disease, with more than 85 million people fully vaccinated in the USA by March 2021 [2]. During the early vaccination period, side effects were reported, ranging from local pain to respiratory failure and death.
Guillain-Barre Syndrome (GBS) is an acute demyelinating inflammatory polyradiculoneuritis, commonly attributable to antecedent infections or other immunogenic events [3]. It occurs in 0.4-4.0 cases per 100,000 people, and less than 1 case of GBS per 1,000,000 immunized people seems to be related to a vaccine [3]. The role of a vaccine triggering GBS has been only proven with the influenza vaccine from 1976 [4].
We present a case of GBS with a temporal relationship to COVID-19 vaccination occurring in a patient who recently underwent spinal surgery and performed a literature review to draw attention to this neurological complication in association with COVID-19 vaccination and/or spinal surgery.
Methods
A literature search was conducted by 2 independent reviewers (MJC and JMO) from PUBMED and Cochrane databases to identify all the relevant studies that have been published in English addressing a temporal relationship between any subtype of COVID-19 vaccination, spinal surgery and GBS. The search terms included were: “COVID-19”, “SARS-CoV-2 vaccination”, “mRNA-based vaccine”, “vector-based vaccine”, “Guillain Barre Syndrome” “Miller- Fisher syndrome”, “side effect “, “adverse reaction”, “spinal surgery”, “Cervical myelopathy” “ACDF” and “polyneuropathy”. Technical notes, literature review, cadaveric studies as well as publications including pathologies distinct from GBS were excluded from the review.
Results
Both reviewers (MJC and JMO) independently screened abstracts and titles after removing 30 duplicate publications. Search results yielded 27 publications reporting 40 patients with a temporal relationship between COVID-19 Vaccination and GBS, and 14 articles reporting 18 patients with a temporal relationship between spinal surgery and GBS. Cases with a temporal relationship of COVID-19 vaccination, GBS and spinal surgery were not found.
Of the 40 patients with GBS after COVID-19 vaccination, 27 received Oxford/Astrazeneca, 3 Johnson&Johnson, 1 CoronaVac and 1 Moderna. Our case report and eight other patients received Pfizer. All the patients except four developed GBS after the first dose. No data is currently available on patients with GBS after a 3rd dose. The mean age was 59.43 years old with a Standard Deviation (SD) of 14.63 and the mean days and SD latency between vaccination and onset of GBS was 11.9 and 6.25 respectively ranging from 1 to 39 days. The treatment included Intravenous Immunoglobulin (IVIGs) (n = 25), Plasmapheresis (PF) (n = 3), IVIGs and PF (n = 6), steroids (n = 3) and different therapy (n = 3). All the cases were reported in 2021.
Of the 18 cases patients with GBS after spinal surgery, 4 patients underwent to posterior thoracolumbar fusion, 1 lumbar fusion, 4 lumbar laminectomy, 1 endoscopic discectomy, 2 cervical laminoplasty, 2 tumoral resections and 1 after kyphoplasty. Our case report and 2 other cases underwent ACDF surgery. The average age was 56.66 years and the SD between patients was 10.08 years. The mean latency between surgery and onset of GBS was 7.40 days with a SD of 6.7 days and ranged from 3 hours to 25 days. The treatment included IVIGs (n = 11), PF and steroids (n = 1), IVIGs and steroids (n = 2), PF and steroids (n = 1), IVIG and steroids (n = 1), IVIGs and PF (n = 1) and no therapy (n = 1). The cases were reported from 1990 to 2018.
To the best of our knowledge, this is the only reported case of GBS with a temporal relationship to COVID-19 vaccination, occurring in a patient who recently underwent spinal surgery.
Case Description
A 65-year-old male, one-month postoperative C4-C5 Anterior Cervical Discectomy and Fusion (ACDF) for cervical myelopathy, was admitted to the Emergency Department for rapidly progressing weakness and paresthesias to both upper and lower extremities. There were no complications related to his ACDF and his myelopathy was initially improving post-operatively. The patient was then evaluated 2 weeks after surgery, he was asymptomatic, and his physical exam was unremarkable. He denied recent travel, gastrointestinal symptoms nor trauma; however, 9 days before his second admission, he had had a single dose of the Pfizer COVID-19 vaccine.
On examination, there were no mental status or cranial nerve abnormalities. His strength was 4/5 on both upper and lower extremities in addition to areflexia and urinary retention. His sensation was intact. Within forty-eight hours from admission, the patient progressed to severe quadriparesis and respiratory failure; therefore, endotracheal intubation was performed.
Investigations
Naturally, there was a concern of spinal cord compression given his recent surgery, but MRI did not reveal acute compression or post-surgical complications (Figure 1 and 2). Furthermore, a thoraco-lumbar spine and brain MRI showed no abnormalities. The patient denied a previous history of COVID-19 infection and the Coronavirus Cov-2 PCR on admission was negative.
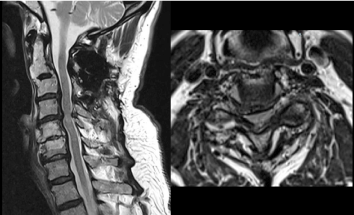
Figure 1: Preoperative MRI (sagittal and axial T2 weighted sequence
without contrast) showing a C4-C5 spinal canal stenosis with a hyperintense
intramedullary signal suggesting myelopathy.
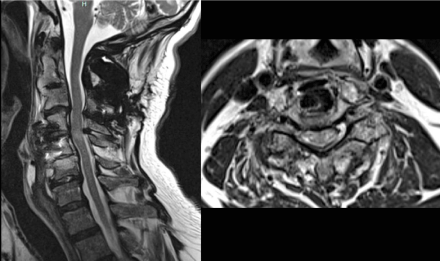
Figure 2: MRI (sagittal and axial T2 weighted sequence without contrast) one
month after surgery without any signs of acute compression or post-surgical
complications.
A diagnosis of GBS was considered due to the rapidly progressive nature of the patient’s presentation, which was supported by Cerebrospinal Fluid (CSF) analysis showing an albumin cytologic dissociation (protein 105 mg/dL and WBC 10/mm3); and GD1 serum antibodies of 177 IV, which are strongly associated with acute motor and sensory neuropathy (AMSAN), an axonal variant of GBS [5,6]. The clinical picture, as well as the CSF analyses and the presence of GD1 serum antibodies, supported a diagnosis of GBS. Unfortunately, a nerve conduction study was not performed.
Differential Diagnoses
Given his recent surgery, the symptoms, and signs of GBS can be confused for those of spinal cord injury secondary to the procedure. The MRI was useful to rule out the presence of postoperative hematoma, ischemia, and displacement of the implant, instability, and infection. In addition, the presence of flaccid quadriparesis and areflexia on the physical examination supported the diagnosis of peripheral neuropathy. However, there was no history of triggers and the basic peripheral neuropathy screening (Vitamin B12 level, folate level, copper level, VDRL, thyroid hormones, HIV test and protein electrophoresis) was unremarkable. Antinuclear and antineutrophil cytoplasmic antibodies, as well as C3 and C4 levels, were within the normal limits.
Treatment
The patient was admitted to the intensive care unit and closely monitored with regular input from the neurology, neurosurgery and physiotherapy teams. Only two doses (2 g/kg daily) of the IVIG were administered. After an anaphylactic reaction with the second dose of IVIG, the treatment was changed to plasma exchange therapy which was administered on seven occasions with minimal improvement.
Outcome
Although his neurologic symptoms were gradually improving when he was discharged to a rehabilitation center, the patient passed away two months after the admission due to complications associated with his ventilator dependence.
Discussion
Correlation between GBS and common vaccines (HPV, MMR, etc.) has been reported in case reports, but no causation has been demonstrated [7], except with the influenza vaccine in 1976 [4]. Thus, vaccines remain widely authorized as their potential benefits overcome their potential risks.
Less than 12 months from their advent, COVID-19 vaccines have been shown to be safe and effective by manufacturers’ data and findings from large clinical trials [8,9]. Albeit, adverse events have been reported, from mild adverse effects such as local pain, fever, flu symptoms, diarrhea, etc.; to severe cases such as myocarditis, thrombosis, respiratory failure, anaphylaxis, and death [10].
Globally, millions have received COVID-19 vaccines and the results seem to be promising. Sporadic cases of GBS may be temporally associated with COVID-19 vaccination, but causation cannot be determined with case reports. [11] As the knowledge of COVID-19 vaccination grows, cases like this may help identify early neurological, or general complications.
During COVID-19 vaccine clinical trials, 2 cases within the Johnson & Johnson studies developed GBS 10 days after injection; one was in the placebo arm and one was in the active vaccine arm. The fact that one participant of the placebo arm also developed GBS within the same timeline, supports the manufacturer’s argument for a coincidental rather than a causal association [12,13].
Currently, eight cases of GBS have been reported in the literature from the general public after Pfizer COVID-19 vaccination. The first case occurred in December 2020 in the USA in a previously healthy female who received her first dose of Pfizer COVID-19 vaccine and developed her symptoms two weeks after [14]. Five cases were reported after the first dose and two cases after the second dose of Pfizer COVID-19 vaccine [15-21]. Additionally, 27 cases occurred after the first dose of the Oxford/Astrazeneca COVID-19 vaccine [22- 40]. Three cases after Johnson and Johnson, one case after the second dose of CoronaVac vaccine and another case after the second dose of Moderna were also reported (Table 1) [22-40].
Reference
Age (years)/sex
Type of vaccine/Doses number
Intervale vaccine-onset symptoms (days)
History/current COVID
Associated trigger
Treatment
Our case
65/M
Pfizer (1st dose)
9
None
ACDF surgery
IVIG/PF
Waheed et al. 2021 [14]
82/F
Pfizer (1st dose)
14
None
None
IVIG
Ogbebor et al. 2021 [16]
86/F
Pfizer (1st dose)
1
None
None
IVIG
Nishiguchi et al. 2021 [18]
71/M
Pfizer (1st dose)
18
None
None
IVIG
Bouattour et al. 2021 [19]
67/M
Pfizer (1st dose)
7
None
None
IVIG
čenščák et al. 2021 [20]
42/M
Pfizer (1st dose)
14
None
None
IVIG
Hughes et al. 2021 [21]
65/M
Pfizer (1st dose)
2
No clarify
None
IVIG
Razok et al. 2021 [15]
73/M
Pfizer (2nd dose)
20
None
None
IVIG
Scendoni et al. 2021 [17]
82/F
Pfizer (2nd dose)
15
None
None
IVIG
Hasan et al. 2021 [22]
62/F
Oxford/Astrazeneca (1st dose)
11
None
None
IVIG
Patel et al. 2021 [23]
37/M
Oxford/Astrazeneca (1st dose)
21
None
None
IVIG
McKean et al. 2021 [24]
65/M
Oxford/Astrazeneca (1st dose) Vaxzevria
9
None
None
IVIG/MP
Min et al. 2021 [25]
58/M
Oxford/Astrazeneca (1st dose)
3
None
None
Neuropathic drugs
Min et al. 2021 [25]
37/F
Oxford/Astrazeneca (1st dose)
4
None
None
Neuropathic drugs
Azam et. al 2021 [26]
67/M
Oxford/Astrazeneca (1st dose)
15
None
None
IVIG
Allen et al. 2021 [27]
54/M
Oxford/Astrazeneca (1st dose)
16
None
None
MP
Allen et al. 2021 [27]
20/M
Oxford/Astrazeneca (1st dose)
26
None
None
MP
Allen et al. 2021
57/M
Oxford/Astrazeneca (1st dose)
21
None
None
IVIG
Allen et al. 2021 [27]
55/M
Oxford/Astrazeneca (1st dose)
29
None
None
None
Maramattom et al. 2021 [28]
43/F
Oxford/Astrazeneca (1st dose)
10
No clarify
No clarify
IVIG
Maramattom et al. 2021 [28]
67/F
Oxford/Astrazeneca (1st dose)
14
No clarify
No clarify
IVIG/PF
Maramattom et al. 202 1[28]
53/F
Oxford/Astrazeneca (1st dose)
12
No clarify
No clarify
IVIG
Maramattom et al. 2021 [28]
68/F
Oxford/Astrazeneca (1st dose)
14
No clarify
No clarify
IVIG
Maramattom et al. 2021 [28]
70/M
Oxford/Astrazeneca (1st dose)
11
No clarify
No clarify
IVIG
Maramattom et al. 2021[28]
69/F
Oxford/Astrazeneca (1st dose)
12
No clarify
No clarify
IVIG
Maramattom et al. 2021 [28]
69/F
Oxford/Astrazeneca (1st dose)
13
No clarify
No clarify
IVIG/PF
Introna et al. 2021 [29]
62/M
Oxford/Astrazeneca (1st dose)
10
None
None
IVIG
Nasuelli et al. 2021 [30]
59/M
Oxford/Astrazeneca (1st dose)
10
None
None
IVIG
Oo M. et al. 2021 [32]
51/M
Oxford/Astrazeneca (1st dose)
14
None
None
IVIG/PF
Oo M. et al. 2021 [32]
65/F
Oxford/Astrazeneca (1st dose)
7
None
Influenza Vaccine
IVIG
Oo M. et al. 2021 [32]
66/M
Oxford/Astrazeneca (1st dose)
21
No clarify
Influenza Vaccine
IVIG
Finsterer et al. 2021 [33]
32/M
Oxford/Astrazeneca (1st dose)
8
No clarify
No clarify
IVIG/PF
Kanabar G, Wilkinson P. 2021 [35]
61/F
Oxford/Astrazeneca (1st dose)
10
No clarify
No clarify
IVIG
Kanabar G, Wilkinson P. 2021 [35]
56/M
Oxford/Astrazeneca (1st dose)
9
No clarify
No clarify
IVIG
Nasueli et al. 2021 [30]
59/M
Oxford/Astrazeneca (1st dose)
10
No clarify
None
IVIG
Kripalani et al. 2021 [40]
52/F
Oxford/Astrazeneca (1st dose)
11
None
None
IVIG
Tutar et al. 2021 [31]
76/M
Corono VAC (2nd dose)
5
No clarify
No clarify
No Clarify
Prasad et al. 2021
41/M
Johnson and Johnson (1st dose)
12
None
None
IVIG
Morehouse et al. 2021 [36]
49/F
Johnson and Johnson (1st dose)
5
None
No clarify
IVIG/PF
Rossetti et al. 2021 [38]
38/M
Johnson and Johnson (1st dose)
14
No clarify
No clarify
IVIG
Dalwadi et al. 21 [39]
86/F
Moderna (2nd dose)
2
No clarify
No clarify
PF
F: Female; M: Male; MP: Methylprednisolone; IVIG: Intravenous Immunoglobulin; C: Corticosteroids; PF: Plasmaferesis.
Table 1: Summary of Previously Reported Guillain Barré Syndrome after COVID-19 vaccine.
To our knowledge this is the largest literature review of GBS with a temporal relationship with COVID-19 vaccination [33]. At the moment of writing this manuscript, November 2021, 40 cases were reported in the literature.
Furthermore, 130 reports of GBS after Janssen COVID-19 vaccination were received before July 2021 in the Vaccine Adverse Event Reporting System [41-43]. The median time to onset of GBS following vaccination was 13 days [43].
Because our patient underwent surgery one month before the onset of the symptoms, it is important to highlight that surgery has occasionally been associated with the development of GBS across various surgical fields, with a vast majority after spine surgery. Gensicke et al. demonstrated that the relative risk of contracting GBS was 13.1 in surgical patients when compared to the normal population [44]. The pathophysiology of post-surgical GBS is not well understood, but the common pathway involves an autoimmune insult on the nervous system [44]. In our review of the cases reports of GBS, we found 18 cases after spinal surgery with symptoms typically presenting 1-2 weeks postoperatively (Table 2) [22-40]. The temporal relationship of more than 1 month between our patient’s surgery and the development of GBS symptoms, make a causal relationship unlikely based on the current knowledge. However, it remains uncertain if the association of both COVID19-vaccination and surgery made our patient more susceptible to this incident.
Reference
Age (years)/sex
Surgery
Intervale vaccine-onset symptoms (days)
Treatment
Dowling et al. 2018 [45]
53/F
L4-L5 endoscopy disectomy
10 days
IVIG
Oku et al. 2018 [51]
66/M
Laminoplasty C3-C6
13 days
MP/IVIG
Rashid et al. 2017 [52]
62/F
Lumbar descompression L3-L4
12 days
IVIG
Chen et al. 2017 [50]
57/M
Lumbar fusion L3-S1
9 days
MP/IVIG 5 days
Sahai et al. 2016 [54]
52/M
Descompression L4-L5
17 days
IVIG
Boghani et al. 2015 [48]
58/M
Descompression L4-L5
3 hours
IVIG/PF
Boghani et al. 2015 [48]
40/M
Laminectomy L4-L5
1 hour
IVIG/PF
Huang et al. 2015 [47]
50/M
Fusion C0-C2 and ACDF C5-c6
7 days
IVIG
Huang et al. 2015 [47]
53/M
Laminoplasty C3-C6
3 days
IVIG
Huang et al. 2015 [47]
69/M
Fusion T10-l5
2 days
IVIG
Huang et al. 2015 [47]
58/M
ACDF and fusion C4-C7
3 days
IVIG
Battaglia et al. 2013 [46]
73/F
Kyphoplasty
7 days
IVIG
Miscusi et al. 2012 [49]
55/M
C5-C7 chordoma resection
36 hours
IVIG
Gensicke et al. 2012 [44]
70/M
Multiple Spine surgeries
No clarify
No clarify
Cheng et al. 2011
59/F
T1-T3 meningioma resection
6 hours
IVIG
Son et al. 2011 [55]
50/M
T10-L2 fusion
8 days
IVIG
Riebel et al. 1995 [53]
62/F
T12- Sacrum
25 days
IVIG/PF/CE
Stambough et al. 1990 [56]
33/F
T5-L2 fusion scoliosis
8 days
PF/CE
Table 2: Table showing a summary of previously reported Guillain Barré Syndrome cases after Spine Surgery.
Given that COVID-19 vaccination is a mass vaccination campaign, and that the annual incidence of GBS is around 10 to 20 cases per 1 million among adults [2], there is a possibility of GBS occurring by chance [42-43]. However, common causes of GBS were ruled out in our patient. The presence of an increasing number of case reports highlighting the occurrence of GBS occurring after COVID-19 infection and vaccination, and the close temporal relationship between the vaccine and the onset of our patient’s symptoms make it reasonable to at least consider the possibility of a causal-relationship. Further investigation, as well as continued monitoring of the side effects of COVID-19 vaccination may be necessary to provide a better knowledge of this association [16,42,43].
Here, we report the only case of GBS with a temporal relationship to COVID-19 vaccination, occurring in a patient who recently underwent spinal surgery. Our patient’s recent history of spinal surgery was a confounding factor. Our patient clinically improved after surgery, and his postoperative MRI was negative for signs of complications. The classic CSF findings of albumin-cytological dissociation associated with a rapidly progressive sensorimotor polyneuropathy, with respiratory compromise and dysautonomia, confirmed the diagnosis of GBS. This highlights the importance of close follow-up and detailed physical examination to recognize neurological complications associated with COVID-19 vaccination [45-57].
Conclusion
GBS is an uncommon complication following COVID-19 Vaccines which currently seem to be the most efficient and effective means to control Coronavirus disease [26,43]. Because GBS following COVID-19 vaccines is considered an “adverse event of special interest’’ we believe this of the la literature and case report can contribute to further research. In addition, this case highlights the importance of considering other neurological diagnoses when presenting new deficits.
Declaration
The authors certify that they have obtained all appropriate patient consent.
References
- Lu H, Stratton CW, Tang W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. Journal of Medical Virology. 2020; 92: 401-402.
- Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, Mcdonald B. Coronavirus (COVID-19) Vaccinations. Our World In Data. 2021.
- Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. 2019; 15: 671-683.
- Salmon DA, Dudley MZ, Carleton BC. Guillain-Barré Syndrome Following Influenza Vaccines Affords Opportunity to Improve Vaccine Confidence. The Journal of Infectious Diseases. 2021; 223: 355-358.
- Dufour C, Co T-K, Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre Syndrome - A case report, systemic review and implication for vaccine development. Brain, Behavior, & Immunity - Health. 2021; 12: 100203.
- Emilien D, Hugh W. Diagnostic Utility of Autoantibodies in Inflammatory Nerve Disorders. JND. 2015; 2: 107-112.
- Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain- Barre’ syndrome. Vaccine. 2019; 37: 5544-5550.
- National Center for Immunization and Respiratory Diseases. Possible Side Effects after Getting a COVID-19 Vaccine. COVID-19. 2021.
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021; 27: 205-211.
- Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain- Barre’ syndrome. Vaccine. 2019; 37: 5544-5550.
- Lunn MP, Cornblath DR, Jacobs BC, et al. COVID-19 vaccine and Guillain- Barré syndrome: let’s not leap to associations. Brain. 2021; 144: 357-360.
- Bourdette D, Killestein J. Quelling Public Fears about Guillain-Barre Syndrome and COVID-19 Vaccination. Neurology. 2021: 10: 1212/ WNL.0000000000011882.
- Márquez Loza AM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré Syndrome in the Placebo and Active Arms of a COVID-19 Vaccine Clinical Trial: Temporal Associations Do Not Imply. 2021.
- Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus. 2021; 13: e13426.
- Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain- Barré syndrome; first reported case from Qatar. Ann Med Surg (Lond). 2021; 67: 102540.
- Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. IDCases. 2021; 24: e01143.
- Scendoni R, Petrelli C, Scaloni G, Logullo FO. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vaccin Immunother. 2021; 17: 4093-4096.
- Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021; 21: 452.
- Bouattour N, Hdiji O, Sakka S, et al. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci. 2022; 43: 755-761.
- čenščák D, Ungermann L, štetkárová I, Ehler E. Guillan-Barré Syndrome after First Vaccination Dose against COVID-19: Case Report. Acta Medica (Hradec Kralove). 2021; 64: 183-186.
- Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Guillain- Barré syndrome after COVID-19 mRNA vaccination in a liver transplant recipient with favorable treatment response. Liver Transpl. 2022; 28: 143- 137.
- Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. 2021; 14: e243629.
- Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021; 14: e242956.
- McKean N, Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021; 14: e244125.
- Min YG, Ju W, Ha YE, et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J Neuroimmunol. 2021; 359: 577691.
- Azam S, Khalil A, Taha A. Guillain-Barre Syndrome in a 67- year-old Male Post COVID-19 Vaccination (Astra Zeneca). 2021; 9: 424-427.
- Allen CM, Ramsamy S, Tarr AW, et al. Guillain-Barré Syndrome Variant Occurring after SARS-CoV-2 Vaccination. Ann Neurol. 2021; 90: 315-318.
- Maramattom BV, Krishnan P, Paul R, et al. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021; 90: 312-314.
- Introna A, Caputo F, Santoro C, et al. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association?. Clin Neurol Neurosurg. 2021; 208: 106887.
- Nasuelli NA, De Marchi F, Cecchin M, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol Sci. 2021; 22; 4747-4749.
- Tutar NK, Eyigürbüz T, Yildirim Z, Kale N. A variant of Guillain-Barre syndrome after SARS-CoV-2 vaccination: AMSAN. Guillain-Barré-szindróma SARS-CoV-2-vakcinációt követoen. Ideggyogy Sz. 2021; 74: 286-288.
- Oo WM, Giri P, de Souza A. AstraZeneca COVID-19 vaccine and Guillain- Barré Syndrome in Tasmania: A causal link?. J Neuroimmunol. 2021; 360: 577719.
- Finsterer J. Exacerbating Guillain-Barré Syndrome Eight Days after Vector- Based COVID-19 Vaccination. Case Rep Infect Dis. 2021; 2021: 3619131.
- Finsterer J, Scorza FA, Scorza CA. Post SARS-CoV-2 vaccination Guillain- Barre syndrome in 19 patients. Clinics (Sao Paulo). 2021; 76: e3286.
- Kanabar G, Wilkinson P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. 2021; 14: e244527.
- Morehouse ZP, Paulus A, Jasti SA, Bing X. A Rare Variant of Guillain-Barre Syndrome Following Ad26.COV2.S Vaccination. Cureus. 2021; 13: e18153.
- Nasuelli NA, De Marchi F, Cecchin M, et al. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol Sci. 2021; 42: 4747-4749.
- Rossetti A, Gheihman G, O’Hare M, Kosowsky JM. Guillain-Barré Syndrome Presenting as Facial Diplegia after COVID-19 Vaccination: A Case Report. J Emerg Med. 2021; 61: e141-e145.
- Dalwadi V, Hancock D, Ballout AA, Geraci A. Axonal-Variant Guillian-Barre Syndrome Temporally Associated With mRNA-Based Moderna SARS-CoV-2 Vaccine. Cureus. 2021; 13: e18291.
- Kripalani Y, Lakkappan V, Parulekar L, Shaikh A, Singh R, Vyas P. A Rare Case of Guillain-Barré Syndrome following COVID-19 Vaccination. Eur J Case Rep Intern Med. 2021; 8: 002707.
- Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID-19 Vaccines After Reports of Adverse Events Among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021; 70: 1094-1099.
- Reporting requirements for healthcare providers administering COVID-19 vaccines.
- Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of Receipt of the Ad26.COV2.S COVID-19 Vaccine With Presumptive Guillain-Barré Syndrome, February-July 2021. JAMA. 2021; 326: 1606-1613.
- Gensicke H, Datta AN, Dill P, Schindler C, Fischer D. Increased incidence of Guillain-Barré syndrome after surgery. Eur J Neurol. 2012; 19: 1239-1244.
- Dowling JR, Dowling TJ. A Rare Axonal Variant of Guillain-Barré Syndrome following Elective Spinal Surgery. Case Reports in Orthopedics. 2018; 2018: 2384969.
- Battaglia F, Sevy A, Moyse E, Roche P-H. Guillain-Barré syndrome following severe head trauma and spine surgery. Revue Neurologique. 2013; 169: 166- 168.
- Huang S-L, Qi H-G, Liu J-J, Huang Y-J, Xiang L. A Rare Complication of Spine Surgery: Guillain–Barré Syndrome. World Neurosurgery. 2015; 84: 697-701.
- Boghani Z, Livingston AD, Simpson EP, Holman PJ, Grossman RG. Acute Onset of Guillain-Barré Syndrome After Elective Spinal Surgery. World Neurosurgery. 2015; 84: 376-379.
- Miscusi M, Currà A, Rocca CD, Missori P, Petrozza V. Acute motor-sensory axonal neuropathy after cervical spine surgery: Case report. SPI. 2012; 17: 82-85.
- Chen EY, Stratton C, Mercer B, Hohler A, Tannoury TY, Tannoury C. Guillain- Barré Syndrome After Elective Spinal Surgery: Journal of the American Academy of Orthopaedic Surgeons. 2017; 25: 587-593.
- Oku T, Maekawa K. Guillain-Barré syndrome after cervical spine surgery: A case report. JA Clin Rep. 2018; 4: 28.
- Rashid A, Kurra S, Lavelle W. Guillain-Barré Syndrome after Revision Lumbar Surgery: A Case Report. Cureus. 2017.
- Riebel GD, Heller JG, Hopkins L. Guillain Barre Syndrome after an Operation on the Spine. A case Report. The Journal of Bone and Joint Surgery British volume. 1995; 77: 1565-1567.
- Sahai N, Hwang KS, Emami A. Guillain-Barré syndrome following elective spine surgery. Eur Spine J. 2017; 26: 6-8.
- Son DW, Song GS, Sung SK, Kim SH. Guillain-Barre Syndrome Following Spinal Fusion for Thoracic Vertebral Fracture. J Korean Neurosurg Soc. 2011; 50: 464-467.
- Stambough JL, Quinlan JG, Swanson J. Guillain Barre Syndrome Following Spinal Fusion for Adult Scoliosis. Spine. 1989; 15: 45.
- Leung C. Guillain-Barre syndrome should be monitored upon mass vaccination against SARS-CoV-2. Hum Vaccin Immunother. 2021; 17: 2957- 2958.