
Case Report
Austin Neurol & Neurosci. 2023; 6(1): 1028.
Very Rare Incident of Pediatric Onset Multiple Sclerosis Complicated with Status Epilepticus: A Case Report
Calcii Cornelia¹; Aminov David¹*; Sprincean Mariana¹; Marga Semion¹; Anton Paduraru Dana Teodora²; Revenco Ninel¹; Groppa Stanislav¹; Palii Ina¹; Pirtu Lucia¹; Hadjiu Svetlana¹
1Nicolae Testemitanu State University of Medicine and Pharmacy, Chisinau, Republic of Moldova
2Department of Mother and Child Medicine, Grigore T.Popa University of Medicine and Pharmacy Iasi, Romania
*Corresponding author: David Aminov Nicolae Testemitanu State University of Medicine and Pharmacy, Chisinau, Republic of Moldova. Email: david353aminov@gmail.com
Received: August 01, 2023 Accepted: August 23, 2023 Published: August 30, 2023
Abstract
Background: Pediatric Onset Multiple Sclerosis (POMS) is a chronic inflammatory disease in the central nervous system that affects children under 18. Children with POMS experience gradual deterioration leading to increased disability and lower life expectancy [1,2].
The Purpose of the Study: To present a rare case of POMS with an onset at 42 months, emphasizing its significance due to its infrequency in this age group. The hope is to encourage the establishment of specific diagnostic and treatment guidelines for POMS in the near future.
Material and Methods: The evolution of POMS in a 3.6-year-old child who was admitted multiple times to the Hospital of Mother and Child Health Care in the Republic of Moldova during acute MS attacks.
Results: In September 2022, the child received a confirmed POMS diagnosis based on McDonald’s criteria (2017), which was later confirmed in April 2023 through OCB-CSF examination. The April 2023 MRI showed changes in demyelination foci with temporary clinical improvement compared to the previous examination after the last acute attack. The child is currently undergoing five days of plasmapheresis, followed by a tapering regimen of oral steroids. Ongoing MRI monitoring is recommended, with consideration of receiving Disease-Modifying Treatments (DMTs) in Romania, where they are approved.
Conclusions: This rare case highlights the importance of raising awareness about POMS in children. Early detection and intervention could lead to the development of new diagnostic and treatment guidelines. However, the lack of access to DMT drugs for POMS treatment in the Republic of Moldova results in significantly lower survival rates compared to other countries.
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory disease targeting myelinated axons in the central nervous system. It exhibits varying degrees of disability, affecting some mildly while others experience gradual deterioration with heightened disability. More prevalent in women, MS is typically diagnosed in the late 20s to early 30s. Despite slightly reduced life expectancy, proper management is crucial to prevent secondary complications [3].
Pediatric MS, also known as Pediatric-Onset Multiple Sclerosis (POMS), occurs before age 18. Its exact cause remains elusive, but factors such as infectious agents (EBV), environmental influences (vitamin D deficiency), and genetic predisposition (HLA-DR2) have been linked to it. POMS symptoms commonly affect cognition, vision, neuromuscular functions, pulmonary, and the renal system. According to studies, the frequency of POMS in children is 5% of all the patients with MS, whereas the onset of POMS before the age of 10 years is observed in less than 1% of patients [3]. Even though neuroplasticity is present in individuals with POMS, they tend to develop similar levels of disability at a much earlier age compared to those with Adult-Onset MS (AOMS). Consequently, their quality of life is frequently compromised, negatively affecting their education, social life, and physical activities. As a result, POMS must be viewed as a severe and highly disabling disease that comes with significant social costs [4-6].
Case Report
T.N., born on September 30th, 2019, was admitted to the Hospital of Mother and Child Health Care in the Republic of Moldova on 28/04/22 with complaints of vomiting, fatigue, somnolence, and loss of appetite. She had flu-like symptoms for the past 4 days, and previous treatment showed no improvement. On the 7th day of hospitalization, she developed right-side convergent strabismus with peripheral palsy. A cerebral MRI revealed multiple pathological foci in the white matter supra and infra-tentorially (Figure 1-4). A preliminary diagnosis of Acute Disseminated Encephalomyelitis (ADEM) was made, and treatment with I.V methylprednisolone (30 mg/kg for 3 days) followed by an oral steroid taper significantly improved the strabismus.
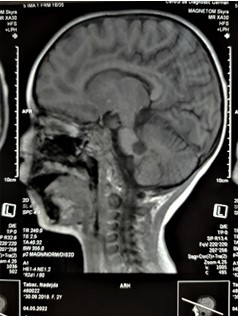
Figure 1: MRI of the brain sagittal view. Hypointense lesions in the right cerebellar peduncles (10x11mm).
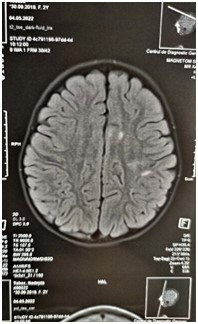
Figure 2: MRI of the brain. Axial view. Left hemisphere hyperintense foci in the basal ganglia.
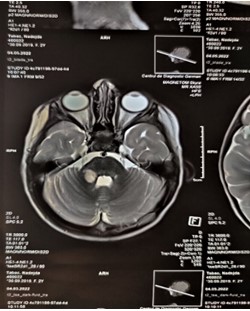
Figure 3: MRI of the brain axial view. Hyperintense lesions foci in the right cerebellar peduncles.
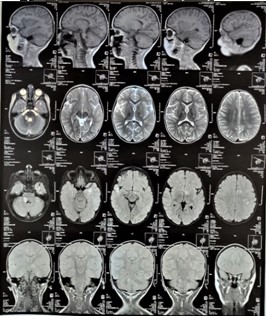
Figure 4: Full T3 MRI of the. Sagittal, axial and coronal views. 4.5.2022.
In July 2022, the patient experienced a paroxysmal event resembling syncope, raising suspicion of an epileptic seizure. An EEG revealed no pathological activities. In August 2022, the patient developed flu-like symptoms followed by right-sided central palsy. A 1.5T cerebral MRI with contrast showed new pathological foci in the left frontal juxtacortical area and an additional focus in the brainstem, with increased foci in the right cerebellar peduncles compared to the previous MRI (Figure 5-7). Periventricular foci size decreased. Ophthalmological examination revealed no abnormalities. IV methylprednisolone 30 mg/kg was administered for three days, but symptoms persisted. Subsequently, IV Immunoglobulins (IVIG) 400 mg/kg were given, resulting in symptom relief.
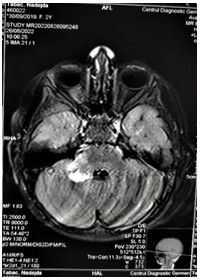
Figure 5: T1 MRI of the brain, Hyperintense foci in the right cerebellar pedicle (18x18mm) and in the left brainstem (8x6mm).
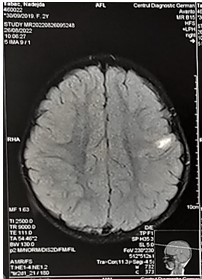
Figure 6: T1 MRI of the brain. Axial view. Hyperintense juxtacortical area on the left (14x7mm).
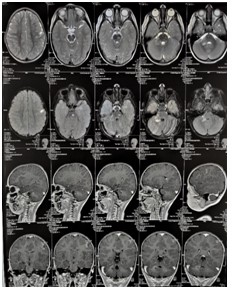
Figure 7: T1 and T2 MRI of the brain. Axial, coronal and sagittal views. 26.08.2022.
Additional MRI of the brain in 1.5T without contrast was scheduled for 30 days later, to evaluate the progression of the MRI findings. The following findings were observed: compared to the previous MRI from 28/8/2022 – there is a progression of the pathological foci formation, due to the appearance of pathological cerebellar focus on the left and an increase of the pathological foci in the right cerebellar pedicle (Figure 8-10).
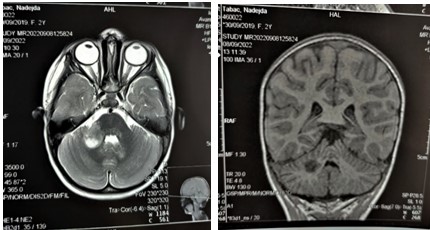
Figure 8,9: MRI of the brain, coronal and axial views. Hyperintense focus in the right cerebellar peduncle (26x18mm).
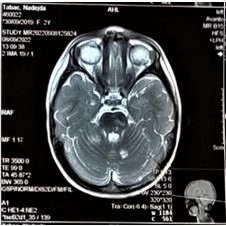
Figure 10: MRI of the brain, axial viewe. Hyperintense focus on the left cerebellar hemisphere (9x9mm).
Due to child's previous two acute attacks and the presence of disseminated opaque lesions identified in last 3 MRI investigations, a diagnosis of POMS was established, according to McDonald's criteria of MS from 2017 with Dissemination In Time and Dissemination In Space (DIT, DIS) (Table 1). To support this diagnosis, the patient underwent full autoimmune markers profiling, and the results of the assessment are as follows:
- = 2 attacks and objective clinical evidence of = 2 lesions.
None. Dissemination in space (DIS) and Dissemination In Time (DIT) have been met.
- = 2 attacks and objective clinical evidence of 1 lesion with historical evidence of prior attack involving lesion in different location.
- = 2 attacks and objective clinical evidence of 1 lesion.
One of the following criteria:
- DIS: additional clinical attack implicating different CNS site.
- DIS: =1 symptomatic or asymptomatic MS-typical T2 lesions in =2 areas of CNS: periventricular, juxtacortical/cortical, infratentorial or spinal cord.
1 attack and objective clinical evidence of 2 or more lesions.
One of the following criteria:
- DIT: additional clinical attack.
- DIT: simultaneous presence of both enhancing and non-enhancing symptomatic or asymptomatic MS-typical MRI lesions.
- DIT: new T2 or enhancing MRI lesion compared to baseline scan (regardless the timing of baseline scan).
- CSF-specific oligoclonal bands.
1 attack and objective clinical evidence of 1 lesion.
One of the following criteria:
- DIS: additional attack implicating different CNS site.
- DIS: =1 MS-typical symptomatic or asymptomatic T2 lesions in =2 areas of CNS: periventricular, juxtacortical/cortical, infratentorial or spinal cord.
AND
One of the following criteria:
- DIT: additional clinical attack.
- DIT: simultaneous presence of both enhancing and non-enhancing symptomatic or asymptomatic MS-typical MRI lesions.
- DIT: by new T2 or enhancing MRI lesions compared to baseline scan (regardless the timing of the baseline scan).
- CSF-specific oligoclonal bands.
Progression from onset
- 1 year of disability progression (retrospective or prospective)
AND
Two of the following criteria:
- =1 symptomatic or asymptomatic MS-typical Y2 lesions (periventricular, juxtacortical/cortical or infratentorial).
- =2 T2 spinal cord lesions.
- CSF specific oligoclonal bands.
Table 1: McDonalds criteria 2017 of multiple sclerosis Thompson et al. 2017.
- Antiphospholipid antibodies IgM-IgG: NEGATIVE (1,2 RU/mL)
- Antinuclear antibodies -immunofluorescent: NEGATIVE (<1/80 titer)
- Anticardiolipin IgG: NEGATIVE (<2 GPL/mL).
- Anticardiolipin IgM: NEGATIVE (2 MPL/mL).
- pANCA: NEGATIVE (<0.2 UI/mL).
- cANCA: NEGATIVE (<0.2 U/mL).
- Extended ANA profile – blot: NEGATIVE.
- Anti-MOG antibodies: NEGATIVE.
- Anti-aquaporin 4 antibodies: <1/10.
Upon conducting an immune-profiling analysis, rheumatoid-autoimmune origin for the acute attacks was excluded from our differentials.
Subsequently, on January 11th 2023, the patient underwent a scheduled MRI checkup in in order to re-assess the progression of the lesion formation. The following findings were found: compared to the previous MRI, there is a regression of the lesions – a decrease in the volume and intensity of the supra- and infratentorial foci (Figure 11,12).
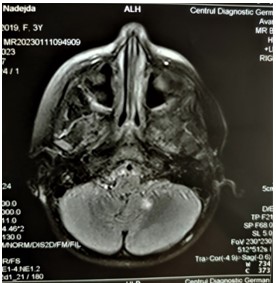
Figure 11: MRI of the brain, axial view. Hyperintense foci in the left cerebellar hemisphere (8 mm).
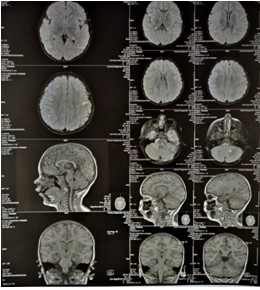
Figure 12: MRI of the brain, axial, coronal and saggital views.
After eight days following that, on 19/01/2023 the patient started to have an ataxic walk and fatiguability. She was hospitalized and on the next day, developed right eye strabismus. A STAT CT scan of the brain was and multiple hyperdense lesion supra and sub-tentorial were found, with impairment of the basal ganglia, insula and midbrain (Figure 13). Following that, the patient was referred to ophthalmological checkup, wherein no pathological abnormalities were found. The patient was prescribed methylprednisolone 30 mg/kg for 3 days following by oral steroid taper for a month, after which the symptoms subsided.
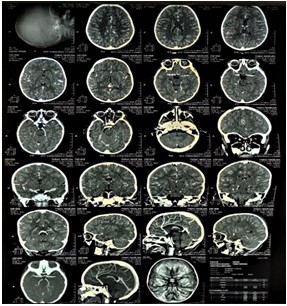
Figure 13: Brian CT. Axial, sagittal and coronal views.
On March 2023 the patient was diagnosed with chickenpox (VZV antibodies IgG, IgM 1,30 ratio and 401,07 IU/I respectively). Three weeks later, the child presented with symptoms of headache, fatigue, and sleepiness. Subsequently, she was admitted to our department for further examination, and within a day of admission, the child reported severe headache and vomiting. Following the incident, an Oligoclonal Bands in the Cerebrospinal Fluid (OCB-CSF) test was indicated and receiving of positive results, further supported our diagnosis of POMS. It is important to note that in addition to OCB-CSF test, a full CSF analysis was done, wherein no abnormalities were detected.
Discussion
Based on available data, approximately 3-5% of MS patients experience the onset before to age of 18 [7-11]. However, it is worth noting the rarity of POMS among younger children is even higher. According research that was conducted at 2003, less than 1% of patients with MS develop the condition before the age of 10, while another research from 2011 found a prevalence of MS in pre-school children (i.e., between 24 months up to 6 years of age), ranging from 0.8 to 14% of POMS cases [3,12]. Our patient presented with central right facial palsy and noticeable ataxic gait at the age of 2.6. In subsequent acute attacks, she developed right-side strabismus and peripheral palsy. She has experienced a total of 3 attacks so far.
Despite extensive research, the exact etiology of POMS remains obscure. Nevertheless, several factors, such as infectious agents like HHV6, EBV and VZV found been linked with the risk of developing MS, particularly due to their ability to remain latent in the body; as well as environmental factors like vitamin D deficiency [12-14], and genetic predisposition such as MHC-I of HLA-DR15, DRB1, DQA1 and DQB1 have been linked with the POMS [15-17]. eWitt et al. found VZV DNA and viral particles in cerebrospinal fluid of 15 MS patients during relapse, suggesting VZV's direct involvement in MS relapses. In March 2023, our child got infected with VZV that was confirmed by blood markers that showed elevated IgM and IgG anti-VZV antibodies a month later (ratio of 1, 3 and 401, 07 IU respectively). Since our child was sick with VZV, the likelihood of her experiencing further relapses in the future has significantly increased. The exceptionally rare occurrence of POMS results in limited recognition and delayed diagnosis. Frequently, there is a tendency for doctors to undertake a thorough, time-intensive and expensive search for metabolic and degenerative disorders other than POMS before considering it as a diagnosis. This often results in a significant delay in diagnosis and treatment for many young patients. Moreover, in cases where patients present with acute symptoms such as confusion, seizures, and cerebrospinal fluid pleocytosis after a viral infection, a diagnosis of meningoencephalitis and ADEM will be frequently established [18]. Our child experienced her first acute POMS attack that accompanied with lesions upon diagnostic imaging, after she got viral infection. Therefore, she was initially misdiagnosed as ADEM, up until the she met the McDonald's diagnostic criteria of MS. As no established protocol exists for managing POMS, treatment approaches often rely on the experience of individual healthcare centers and neurologists, adapting MS therapeutic protocols designed for adults [19]. Glucocorticoids like methylprednisolone and IVIG are used to reduce inflammation and hasten recovery from acute attacks in MS [20], while DMTs such as interferon beta-1a and 1b, fingolimod, or dimethyl fumarate are employed to decrease relapse frequency and the formation of new brain lesions [21-23]. Our patient received methylprednisolone for all acute attacks, and IVIG was administered during the second attack when symptoms were resistant to steroids. However, due to funding and regulatory barriers in the Republic of Moldova, DMTs are not readily available, prompting exploration of alternative countries for treatment. It's important to acknowledge that seeking treatment abroad poses challenges for the patient, including logistical, language, and cultural differences, especially considering her young age.
Conclusions
1. Pediatric onset multiple sclerosis is relatively rare condition that affects children under the age of 10, seen in 2-5 % of all MS cases. Our patient a female, 3.6 years old presented with ambiguous neurological manifestation and underwent series of examinations leading to a definitive diagnosis of POMS. This case is the first one recoded in the Republic of Moldova at this pediatric age group, and one of few recorded worldwide.
2. Diagnosing MS in children can be challenging due to overlapping symptoms and the difficulty children may have in articulating their experience. In our case, it took one year to establish the diagnosis. Through careful analysis we were able to record the condition and deliver data for future scientific research.
Lack of funding and proper regulations in countries like the Republic of Moldova, is limiting the availably of treatment for POMS. Resolving these issues is crucial to provide assistance for individuals with such rare cases.
References
- Luzzio C. Multiple sclerosis. emedicine. Practice Essentials, Background, Pathophysiology. 2023.
- Multiple sclerosis (MS). JHM. 2023. Available from: http://www.hopkinsmedicine.org/health/conditions-and-diseases/multiple-sclerosis-ms.
- Gadoth N. Multiple sclerosis in children. Brain Dev. 2003; 25: 229-32.
- Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol. 2018; 18: 27.
- McKay KA, Ernstsson O, Manouchehrinia A, Olsson T, Hillert J. Determinants of quality of life in pediatric- and adult-onset multiple sclerosis. Neurology. 2020; 94: e932-41.
- McKay KA, Hillert J, Manouchehrinia A. Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. 2019; 92: e2764-73-e2773.
- Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D, University of British Columbia MS Clinic Neurologists. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002; 59: 1006-10.
- Duquette P, Murray TJ, Pleines J, Ebers GC, Sadovnick D, Weldon P, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr. 1987; 111: 359-63.
- Bigi S, Banwell B. Pediatric multiple sclerosis. J Child Neurol. 2012; 27: 1378-83.
- Belman AL, Krupp LB, Olsen CS, Rose JW, Aaen G, Benson L, et al. Characteristics of children and adolescents with multiple sclerosis. Pediatrics. 2016; 138: e20160120.
- Chitnis T, Glanz B, Jaffin S, Healy B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult Scler. 2009; 15: 627-31.
- Ruggieri M, Plasmati I, Simone I. Epidemiology of pediatric multiple sclerosis: incidence, prevalence, and susceptibility risk factors. In: Chabas D, Waubant EL, editors. Demyelinating disorders of the central nervous system in childhood. Cambridge: Cambridge University Press. 2011; 19-35.
- Ascherio A, Munch M. Epstein-Barr virus and multiple sclerosis. Epidemiology. 2000; 11: 220-4.
- Haahr S, Plesner AM, Vestergaard BF, Höllsberg P. A role of late Epstein-Barr virus infection in multiple sclerosis. Acta Neurol Scand. 2004; 109: 270-5.
- International Multiple Sclerosis Genetics Consortium, Wellcome Trust Case Control Consortium 2, Sawcer S, Hellenthal G, Pirinen M, Spencer CC et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011; 476: 214-9.
- Patsopoulos NA, Barcellos LF, Hintzen RQ, Schaefer C, van Duijn CM, Noble JA, et al. Fine-mapping the genetic association of the major histocompatibility complex in multiple sclerosis: HLA and non-HLA effects. PLOS Genet. 2013; 9: e1003926.
- Banwell BL. Pediatric multiple sclerosis. Curr Neurol Neurosci Rep. 2004; 4: 245-52.
- McKay KA, Manouchehrinia A, Berrigan L, Fisk JD, Olsson T, Hillert J. Long-term cognitive outcomes in patients with pediatric-onset vs adult-onset multiple sclerosis. JAMA Neurol. 2019; 76: 1028-34.
- Gadoth N. Multiple sclerosis in children. Brain Dev. 2003; 25: 229-32.
- Margoni M, Rinaldi F, Perini P, Gallo P. Therapy of pediatric-onset multiple sclerosis: state of the art, challenges, and opportunities. Front Neurol. 2021; 12: 676095.
- Lotze E, Timothy E, Francisco . González Scarano. UpToDate. Treatment and prognosis of pediatric multiple sclerosis Marc, editor; C Patterson. 2023. Available from: http://www.uptodate.com/contents/treatment-and-prognosis-of-pediatric-multiple-sclerosis?search=pediatric+multiple+sclerosis&source=search_result&selectedTitle=2~20&usage_type=default&display_rank=2.
- Narula S, Hopkins SE, Banwell B. Treatment of pediatric multiple sclerosis. Curr Treat Options Neurol. 2015; 17: 336.
- Yeh EA. Current therapeutic options in pediatric multiple sclerosis. Curr Treat Options Neurol. 2011; 13: 544-59.