
Research Article
Austin Neurol & Neurosci. 2023; 6(1): 1029.
Augmented Glycemic Gap is a Marker for Predicting the Early Neurological Outcomes in Acute Ischemic Stroke Patients Treated with Intravenous Thrombolysis
Ling Wang1#; Nuo Wang1#; Ting Hu2#; Haiyan Liu2; Tao Wu3*; Qiantao Cheng4*
1Department of Neurology, Changhai Hospital, Second Military Medical University, Shanghai, China
2Department of Neurology, The Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
3Center of Cerebrovascular Disorders, Department of Neurology, Second Military Medical University, Shanghai, China
4Department of Neurology, Huai’an 82 hospital, Jiangsu, China
#These authors have contributed equally to this article.
*Corresponding author: Tao Wu Center of Cerebrovascular Disorders, Department of Neurology, Second Military Medical University, Shanghai, China; Qiantao Cheng, Department of Neurology, Huai’ an Hospital, Jiangsu, China. Tel: (+)86-13391232009; (+)86-13033553377 Email: twu163@163.com; hongtao20030706@126.com
Received: August 23, 2023 Accepted: September 21, 2023 Published: September 28, 2023
Abstract
Background and Purpose: Glycemic Gap (GG) as an index reflecting the acute fluctuation of glycemia, has been proved to be associated with poor functional outcomes in Acute Ischemic Stroke (AIS) patients. However, it predictive value on post-thrombolysis Early Neurological Outcomes (ENOs) are still controversial. This study aimed to use GG to evaluate the influence of pretreatment relative glucose changes on post-thrombolysis ENOs, and we further explored the predictive value of GG in different glycemic control status.
Methods: Early Neurological Deterioration (END) was defined as a National Institutes of Health Stroke Scale Score (NIHSS) =4, Early Neurological Improvement (ENI) was defined as a =4-point decrease in NIHSS score or a complete resolution of neurological deficits, between the time of admission and 24 hours after intravenous recombinant tissue-type plasminogen activator (IV-rtPA). GG was calculated as Admission Blood Glucose level (ABG)- estimated average blood glucose level (eAG), eAG could be derived from HbAlc according to the equation eAG=28.7*HbAlc-46.7
Results: Increased GG was significantly associated with post-thrombolysis END and poor functional outcome at discharge (OR, 1.982; 95% CI, 1.213-3.238; P=0.006) (OR, 2.079; 95% CI, 1.305-3.312; P=0.002). Its predictive value on END was more pronounce in diabetic patients (OR, 2.434; 95% CI, 1.079-5.491; P=0.032), after dichotomizing glycemic control status, its significance was only maintained in diabetic patients with good previous glucose control (OR, 6.946; 95% CI, 1.217-39.636; P=0.029).
Conclusion: An evaluated GG was associated with high risk of post-thrombolysis END and poor functional outcome at discharge in AIS patients, and the previous glucose control should be considered when predicting ENOs.
Keywords: Acute ischemic stroke; Early neurological deterioration; Poor functional outcome; Glycemic gap; Stress induced hyperglycemia
Introduction
Stress-Induced Hyperglycemia (SIH) is a common phenomenon during acute phase of severe illness, it could be a hallmark of diseases severity [1]. SIH controlled background glycemia reflects the fluctuation of glucose in the acute phase of the diseases. The underlying mechanisms involves activation of hypotha-lamic-pituituary-adrenal axis and sympatho-adrenal system, reflects the severity of physiological stress [2]. Glycemia Gap (GG) is a novel index of glycemic excursion to quantifies the relative glycemic rise from chronic glycemia in the acute phase of illness. Previous studies have confirmed that GG is a useful marker for predicting poor outcomes after some critical illness, including ischemic stroke [3-5]. However, the prognostic value of GG requires comprehensive consideration patients previous glucometabolic level. The deleterious effects of increased GG was more pronounce in diabetic patients [6], according to the results of one study, the effect of elevated GG on poor functional outcome was more significant in diabetic patients with good previous glucose control [3].
Early Neurological Deterioration (END) after Acute Ischemic Stroke (AIS) is a prominent clinical issue that is strongly correlated with poor functional outcome and mortality [7,8]. In our previous study, we found that Stress Hyperglycemia Ratio (SHR), which is calculated as Admission Blood Glucose (ABG) level divided by glycosylated hemoglobin (HbAlc %), was significantly associated with END, and this association is more pronounced in diabetic patients [9]. Compared with SHR, the GG was calculated as ABG minus the estimating blood glucose (eAG) determined by HbAlc [10], reflecting the absolute difference from eAG [11]. The predictive value of GG on END is unclear. Therefore, the aim of this study was to validate its predictive value on Early Neurological Outcomes (ENO), and we further explored the whether the predictive effects is different in AIS patients with different previous glucose metabolism.
Method
Study Population
This study retrospectively included consecutive patients with AIS treated with intravenous recombinant tissue-type plasminogen activator (IV-rtPA) at the Department of Neurology, Center of Cerebrovascular Disorders, ChangHai Hospital and Department of Neurology, The Second Affiliated Hospital of Xu Zhou Medical School from January 2017, to December 2020. Patients enrolled in this study if they: (1) aged 18 years or older; (2) were admission within 4.5h after onset; and (3) were treatment with IV-rtPA. Patients were excluded from this study if they: (1) were diagnoses of malignant tumors, autoimmune diseases, major organ failure or presence of an active infection; and (2) had incomplete clinical data. We further excluded patients treated with IV-rtPA and endovascular thrombectomy to maintain the homogeneity of the enrolled patients. Written informed consent was obtained from participants or legal representatives. The study protocol was approved by Ethics Committee of ChangHai Hospital and The Second Affiliated Hospital of Xu Zhou Medical School.
Baseline Assessments
Stroke severity was assessed via National Institutes of Health Stroke Scale (NIHSS). The Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria was used to classified stroke subtype [12]. The diagnosis of Symptomatic Intracranial Hemorrhage (sICH) was based on the results of CT scans, combined with a NIHSS score of =4. A poor functional outcome at discharge was defined as a Modified Rankin Scale (mRS) score of 3-6 at discharge. The mRS score were collected the day before discharge by two trained physicians independent of the study. The proximal artery occlusion confirmed by the Computed Tomography Angiography (CTA), Magnetic Resonance Angiography (MRA) and Digital Subtraction Angiography (DSA). Image data were reviewed in blind manner by two physicians, with advice of the third experienced physicians in case of disagreement.
Definition of Post-Thrombolysis Early Neurological Outcome
The post-thrombolysis END was defined as a =4-point increase in NIHSS score between the time of admission and 24 hours after IV-rtPA [13]. Meanwhile post-thrombolysis ENI was defined as a =4-point decrease in NIHSS score or a complete resolution of neurological deficits between the time of admission and 24 hours after IV-rtPA [14,15]. Neurological deficit was evaluated on admission and at 24 hours after IV-rtPA by two certified neurologists blinded to the clinical data.
Assessment of Glycemic Gap
Fasting plasm glucose levels were monitored shortly after admission before IV-rtPA. Glycosylated hemoglobin (HbAlc%) was measured within 24 hours after hospitalization, estimated average blood glucose level (eAG) could be derived from HbAlc according to the equation eAG=28.7*HbAlc-46.7 [10]. GG was calculated as ABG-eAG [11].
Assessment of Abnormal Glucose Metabolism Status
According to the recommended of American Diabetes Association (ADA), DM was diagnosed based on prior history of diabetes or an HbAlc =6.5% and patients with HbAlc less than 5.7% were classified as NGM [16]. Diabetic patients were classified into 2 groups according to PGC, diabetic patients with good PGC had HbAlc <7%, diabetic patients with poor PGC had HbAlc =7% [16]. Patients HbAlc was measured within 24 hours after hospitalization. Admission blood glucose was measured shortly after arrived the emergency room, hyperglycemia was defined as blood glucose levels higher than 7.8mmol/L [17].
Statistical Analysis
The Kolmogorov–Smirnov test was performed to test the normality of variables, and continuous variables were described as the mean (standard deviation) and median (quartile) based on the normality of the data. Categorical variables are expressed as percentages. Differences in the baseline characteristics were assessed by Χ² test for categorical variables and ANOVA or Kruskal–Wallis test for continuous variables.
Multivariate logistical regression was performed to analysis the association of GG and early neurological outcome, patients were tertiled according to the GG value, the first tertile group as the reference category. The covariates entered in the multivariable logistical regression were age, gender admission NIHSS, Neutrophil to Lymphocyte Ratio (NLR), time of Onset To Treatment (OTT), proximal artery occlusion, stroke subtype, sICH. The Receiver Operating Characteristic (ROC) curve was used to determine the accuracy of GG in predicting ENOs in AIS patients treated with IV-rtPA, two-tailed P values of <0.05 were considered statistically significant. Data analyses were performed using the statistical software package SPSS 22.0 for Windows (IBM, Armonk, NY).
Results
Baseline Characteristics
A total of 798 AIS patients treated with IV-rtPA were included in this study, 139(17.4%) patients had END, 207(25.9%) patients had ENI and 215(26.9%) patients had poor function outcome. Patients were tertiled according to the GG value, the baseline characteristics were presented in table 1. Patients in the higher GG group were more likely to have higher systolic blood pressure (151 vs. 156 vs.153; P=0.019); to have a higher incidence of large artery atherosclerosis and DM (23.3% vs. 28.6% vs. 36.8%; P=0.041) (37.2% vs. 19.5% vs. 48.1%; P<0.001); to have a higher occurrence of END and poor functional outcome at discharge (13.2% vs. 14.3% vs. 24.8%; P<0.001) (11.7% vs. 16.5% vs. 52.6%; P<0.001); to have a higher level of NLR (2.3 vs. 2.4 vs. 2.8; P<0.001). Patients were less likely to experience post-thrombolysis ENI in the higher GG tertile group (31.2% vs. 26.3% vs. 20.3%; P=0.016).
Table 1: Baseline Characteristics of subgroups based on the tertile of glycemic gap.
In table 2, Patients were divided into non-diabetic and diabetic groups, in both groups patients in the higher GG group have a higher risk of END and poor functional outcome at discharge (12.1% vs. 12.1% vs. 22.0%; P=0.014) (12.9% vs. 21.5% vs. 29.0%; P=0.026) (19.1% vs. 20.2% vs. 34.7%; P=0.001) (22.6% vs. 30.1% vs. 40.1%; P=0.026). In the diabetic group, patients more likely to experienced ENI in the lower GG group (31.2% vs. 29.0% vs. 15.1%; P=0.026).
Non-diabetic
Diabetic
T1
(n=173)T2
(n=173)T3
(n=173)P
T1
(n=93)T2
(n=93)T3
(n=93)P
Clinical outcomes
END, n (%)
21 (12.1)
21 (12.1)
38 (22.0)
0.014
12 (12.9)
20 (21.5)
27 (29.0)
0.026
ENI, n (%)
48 (27.7)
45 (26.0)
44 (25.4)
0.879
29 (31.2)
27 (29.0)
14 (15.1)
0.022
Poor outcome, n (%)
33 (19.1)
35 (20.2)
60 (34.7)
0.001
21 (22.6)
28 (30.1)
38 (40.1)
0.026
END: Early Neurological Deterioration; ENI: Early Neurological Improvement; P for trend
Table 2: Clinical outcomes of subgroups based on the presence diabetes mellitus.
Association of GG and Early Neurological Outcomes in Multivariate Analysis
Multivariate logistical regression was performed to analyze the relationship between GG and early neurological outcomes for all patients and for the subgroups of patients with different states of glucose metabolism. The results are showed in table 3,4 and 5. After adjusting for confounders, the risk of END and poor functional outcome at discharge was significantly higher in third tertile group than those of patient in the first tertile group (as the reference value) (OR, 1.982; 95% CI, 1.213-3.238; P=0.006) (OR, 2.079; 95% CI, 1.305-3.312; P=0.002). However, the probability of ENI is significantly lower in the third tertile group (OR, 0.492; 95% CI, 0.322-0.752; P=0.010). In the subgroups analysis, the predictive effect of increased GG on END and ENI was significant only for diabetic patients (OR, 2.434; 95% CI, 1.079-5.491; P=0.032) (OR, 0.374; 95% CI, 0.173-0.806; P=0.012). However, augmented GG was significantly associated with poor functional outcome at discharge in both the non-diabetic and diabetic groups (OR, 2.536; 95% CI, 1.337-4.812; P=0.004) (OR, 2.423; 95% CI, 1.099-5.344; P=0.028). Table 5 illustrated that predictive value of increased GG on END was more pronounce in diabetic patients with good previous glucose control (good-PGC) (OR, 6.946; 95% CI, 1.217-39.636; P=0.029), however, its effect on ENI was significant both in diabetic patients with poor previous blood glucose control (poor-PGC) and good-PGC (OR, 0.182; 95% CI, 0.051-0.646; P=0.008) (OR, 0.283; 95% CI, 0.081-0.993; P=0.049).
outcomes
GG
OR
95% CI
P
END
T1
Ref
T2
1.034
0.604-1.768
0.904
T3
1.982
1.213-3.238
0.006
ENI
T1
Ref
T2
0.761
0.512-1.129
0.175
T3
0.492
0.322-0.752
0.010
Poor outcome
T1
Ref
T2
0.981
0.594-1.618
0.939
T3
2.079
1.305-3.312
0.002
END: Early Neurological Deterioration; ENI: Early Neurological Improvement; GG: Glycemic Gap; OR: Odd Ratio; CI: Confident Interval; P for trend.
Table 3: Laboratory values at hospital admission.
Table 4: Multivariate logistical regression analyses depicting the association of GG and respective early neurological outcomes in patients with and without DM.
DM
Good-PGC
Poor-PGC
GG
OR
95%CI
P
OR
95% CI
P
END
T1
Ref
Ref
Ref
Ref
T2
2.690
0.417-17.346
0.298
1.542
0.620-3.839
0.352
T2
6.946
1.217-39.636
0.029
1.511
0.617-3.705
0.367
ENI
T1
Ref
Ref
Ref
Ref
T2
0.672
0.225-2.003
0.475
1.266
0.534-2.998
0.592
T3
0.283
0.081-0.993
0.049
0.182
0.051-0.646
0.008
DM: Diabetes Mellitus; END: Early Neurological Deterioration; ENI: Early Neurological Inprovement; GG: Glycemic Gap; PGC: Previous Glucose Control; OR: Odd Ratio; CI: Confident Interval; P for trend.
Table 5: Multivariate logistical regression analyses depicting the association of GG and respective early neurological outcomes in DM patients with different previous glucose ccontrol.
ROC Curves were used to Test the Overall Discriminative Ability of GG for Early Neurological Outcomes in DM with Different PGC Status
ROC curves were presented in Figure1, 2. We founded that the Area Under Curve (AUC) of GG to discriminate post-thrombolysis Early Neurological outcomes (END and ENI) in DM patients with good-PGC and poor-PGC were 0.741 (95% CI, 0.621-0.862), 0.550 (95% CI, 0.460-0.640), 0.628 (95% CI, 0.510-0.746), 0.609 (95% CI, 0.519-0.699). The optimal cutoff values of GG on post-thrombolysis END and ENI in DM with different PGC was established based on the highest Youden index, the results were described in additional file1: table S1.
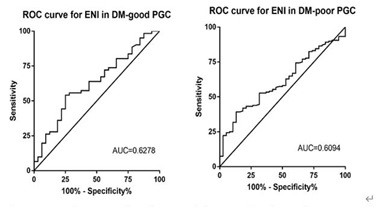
Figure 1: Receiver operating characteristic curve for glycemic gap to predict post-thrombolysis ENI in DM patients with different previous glucose control status.
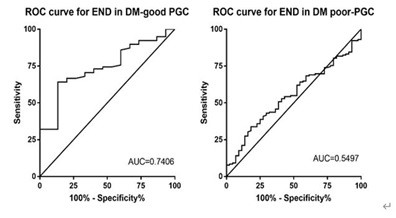
Figure 2: Receiver operating characteristic curve for glycemic gap to predict post-thrombolysis END in DM patients with different previous glucose control status.
Discussion
This study indicated that the augment GG has adverse effect on early neurological outcomes in AIS patients treated with IV-rtPA. Moreover, we found that the deleterious effect of increased GG differ among different glucose metabolism status, the predictive effect on early neurological outcomes was more pronounced in diabetic patients, and after dichotomizing PGC status, its significance for post-thrombolysis END was only maintained in DM with good-PGC group.
According to this study, we confirmed the adverse effects of increased GG, and compared with our previous study, we used ABG level divided by glycosylated hemoglobin (HbAlc %) to represent stress-induced hyperglycemia [9], the value of GG can better reflect absolute changes of glycemia by controlling for background glycemia. Previous studies have demonstrated that GG was significantly associated with worse outcome in critically ill patients with DM [18]. In patients with acute ischemic stroke, GG might be useful in assessing disease severity and prognosis [11]. The underlying mechanisms between increased GG and END is unclear, possible explanations are acute stress response involves activation of the hypothalamic-pituitary axis and sympatho-adrenal system and promote the releases of epinephrine, norepinephrine and pro-inflammatory cytokines (TNF-a, IL-1 and IL-6) [2]. The greater inflammatory and neurohormonal mediates the breakdown of Blood-Brain Barrier (BBB), increased the risk of brain edema and hemorrhage after reperfusion [19]. Pro-inflammatory environment and hyperglycemia may facilitate the glucose uptake in center nervous system, cellular glucose overload increases brain lactate production and further promotes the conversion of asymptomatic tissue to symptomatic tissue [20]. In addition, the increased GG reflects greater glycemic variability in AIS patients treated with IV-rtPA, previous study have shown the greater glycemic variability in AIS patients may be correlated with lower odds of ENI during hospitalization [21]. The specific triggering effect of glucose fluctuation on oxidative stress is likely to be the culprit [22].
In this study, we found the AIS patients with diabetes were more prone to experienced post- thrombolysis END, especially in those with good previous glucose control. This conclusion is consistent with previous studies reporting that admission GG was associated with poor stroke prognosis, and its predictive effect seems more significant in DM with good-PGC [3,11]. The exact pathomechanism is undetermined, possible explanations are that chronic hyperglycemia was associated with vascular remodeling and influence collateral circulation [23], AIS patients with diabetic microangiopathy were more sensitivity to ischemia and hypoxia, the over consumption of intracellular oxygen results in deepening and an extension of hypoperfusion. In addition, hyperglycemia and hyperinsulinemia can decrease the fibrinolytic activity by increasing the production of plasminogen activator inhibitor, further affects the activity of rt-PA and impaired recanalization after AIS [24]. In diabetic patients, the risk of vascular complications and death were strongly associated with previous glycemic exposure [25], the glucose fluctuation may be more common phenomenon in poor-PGC group, therefore, when suffering an acute stress, the tolerance mechanisms may be established to some extent in those patients, oxidative stress and inflammatory response may be relatively mild than DM with good-PGC. Moreover, diabetic patients with poor-PGC have a higher baseline glucose level, the threshold GG value of affecting prognosis might be more higher than good-PGC group.
The present study has several potential limitations that should be addressed when interpreting the results. First, this is a retrospective study; approximately one-fifth of the patients were excluded because they lacked HbAlc values, and we excluded patients treated with endovascular therapy after IV-rtPA, which inevitably produced biases. Second this study did not complete pre-stroke medication information, especially the use of hypoglycemic drug. Third, this study was performed in a single country, which might limit the generalizability of the results to other patient cohorts. However, the strengths of this study include the large sample size and the fact that patients were selected from multiple centers via strict inclusion and exclusion criteria.
Conclusion
In conclusion, the augment GG have a predictive value on post-thrombolysis ENO, and the previous glycemia control status will influence the predictive value of GG. This conclusion suggests that GG might become an important modifiable therapeutic target in prevention of post-thrombolysis ENO and poor functional outcome.
Author Statements
Statement of Ethics
This study was conducted according to the protocol approved by the Human Subjects Research Ethics Committee of ChangHai Hospital and The Second Affiliated Hospital of Xu Zhou Medical School.
Conflict of Interest
The authors declare no competing interests.
Acknowledgements
We thank all patients for their participant in this study.
Author Contributions
Tao Wu and Qiantao Cheng designed the study. Ling Wang, Nuo Wang, Ting Hu conducted the research, JingJing Chen, Xin Gao and ChunHua Feng Assist with data collection. Ling Wang wrote the paper and analyzed the data. All authors reviewed and approved the final version of the manuscript.
References
- Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015; 100: 4490-7.
- Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013; 17: 305.
- Kim Y, Lee SH, Kim C, Kang MK, Yoon BW, Kim TJ et al. Personalized consideration of admission-glucose gap between estimated average and initial glucose levels on short-term stroke outcome. J Pers Med. 2021; 11: 139.
- Wang C, Wang W, Li G, Wang A, Zhang X, Xiong Y et al. Prognostic value of glycemic gap in patients with spontaneous intracerebral hemorrhage. Eur J Neurol. 2022; 29: 2725-33.
- Zhu Y, Liu K, Meng S, Jia R, Lei X, Chen M, et al. Augmented glycaemic gap is a marker for an increased risk of post-infarct left ventricular systolic dysfunction. Cardiovasc Diabetol. 2020; 19: 101.
- Chen PC, Tsai SH, Wang JC, Tzeng YS, Wang YC, Chu CM, et al. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLOS ONE. 2019; 14: e0223126.
- Dávalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999; 30: 2631-6.
- Mori M, Naganuma M, Okada Y, Hasegawa Y, Shiokawa Y, Nakagawara J et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the Stroke Acute Management with Urgent Risk Factor Assessment and Improvement rt-PA Registry. Cerebrovasc Dis. 2012; 34: 140-6.
- Wang L, Cheng Q, Hu T, Wang N, Wei X, Wu T, et al. Impact of stress hyperglycemia on early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis. Front Neurol. 2022; 13: 870872.
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008; 31: 1473-8.
- Yang CJ, Liao WI, Wang JC, Tsai CL, Lee JT, Peng GS, et al. Usefulness of glycated hemoglobin A1c-based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am J Emerg Med. 2017; 35: 1240-6.
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993; 24: 35-41.
- Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015; 86: 87-94.
- Tian C, Ji Z, Xiang W, Huang X, Wang S, Wu Y et al. Association of lower leukocyte count before thrombolysis with early neurological improvement in acute ischemic stroke patients. J Clin Neurosci. 2018; 56: 44-9.
- Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021; 18: 51.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36: S67-74.
- Zuurbier SM, Hiltunen S, Tatlisumak T, Peters GM, Silvis SM, Haapaniemi E, et al. Admission hyperglycemia and clinical outcome in cerebral venous thrombosis. Stroke. 2016; 47: 390-6.
- Lou R, Jiang L, Zhu B. Effect of glycemic gap upon mortality in critically ill patients with diabetes. J Diabetes Investig. 2021; 12: 2212-20.
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001; 21: 1393-400.
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002; 52: 20-8.
- Palaiodimou L, Lioutas VA, Lambadiari V, Theodorou A, Themistocleous M, Aponte L, et al. Glycemic variability of acute stroke patients and clinical outcomes: a continuous glucose monitoring study. Ther Adv Neurol Disord. 2021; 14: 17562864211045876.
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295: 1681-7.
- Hou Q, Zuo Z, Michel P, Zhang Y, Eskandari A, Man F, et al. Influence of chronic hyperglycemia on cerebral microvascular remodeling: an in vivo study using perfusion computed tomography in acute ischemic stroke patients. Stroke. 2013; 44: 3557-60.
- Pandolfi A, Iacoviello L, Capani F, Vitacolonna E, Donati MB, Consoli A. Glucose and insulin independently reduce the fibrinolytic potential of human vascular smooth muscle cells in culture. Diabetologia. 1996; 39: 1425-31.
- Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012; 55: 636-43.