Review Article
Austin Neurosurg Open Access. 2014;1(2): 1006.
Decompressive Craniectomy for Poor-Grade Aneurysmal Subarachnoid Hemorrhage
Naoki Otani*, Kojiro Wada and Kentaro Mori
Department of Neurosurgery, National Defense Medical College, Japan
*Corresponding author: Naoki Otani, Department of Neurosurgery, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan
Received: April 05, 2014; Accepted: May 05, 2014; Published: May 06, 2014
Abstract
Patients with poor-grade aneurysmal subarachnoid hemorrhage (SAH) frequently suffer devastating sequelae caused by the primary and secondary impacts on the brain, particularly if associated with large intracerebral hematoma, sylvian hematoma, or acute subdural hematoma, which result in poor outcomes due to the significant brain stem compression caused by the mass effect. Decompressive craniectomy (DC) is known to reduce the morbidity and mortality in critically ill patients with massive ischemic infarction and severe head injury. However, the role of DC in the treatment of SAH patients is not fully understood. Several experimental studies have indicated that DC significantly improves outcome due to increased intracranial pressure or reduced perfusion pressure. Clinical reports about the efficacy of DC for poor–grade aneurysmal SAH are reviewed here.
Keywords: Brain stem compression; Massive ischemic infarction; Subarachnoid hemorrhage; Cerebral angiography; Intracranial hypertension
Introduction
Poor–grade aneurysmal subarachnoid hemorrhage (SAH) is a frequently devastating condition due to the primary and secondary impacts on the brain, particularly if associated with large intracerebral hematoma (ICH), sylvian hematoma, or acute subdural hematoma (ASDH), which result in poor outcomes due to the significant brain stem compression caused by the mass effect. Decompressive craniectomy (DC) is known to reduce the morbidity and mortality in critically ill patients with massive ischemic infarction [1–4] and severe head injury [5,6]. However, the role of DC in the treatment of poor–grade SAH remains obscure. Recent studies suggested that DC with dural plasty intended to enlarge the intracranial space allows the swollen cerebral hemisphere to expand out of the normal cranial limits, thus avoiding progression of brain herniation, which results in both improvement of cerebral compliance and decrease in intracranial pressure (ICP), and rises in both cerebral blood flow and cerebral microvascular perfusion, possibly accompanied by elevation in brain tissue oxygen [7–9]. Therefore, DC might be an effective strategy for poor–grade SAH [10–12]. The present study reviewed experimental and clinical reports about the efficacy of DC for patients with poor–grade aneurysmal SAH.
Representative Case and Case Series
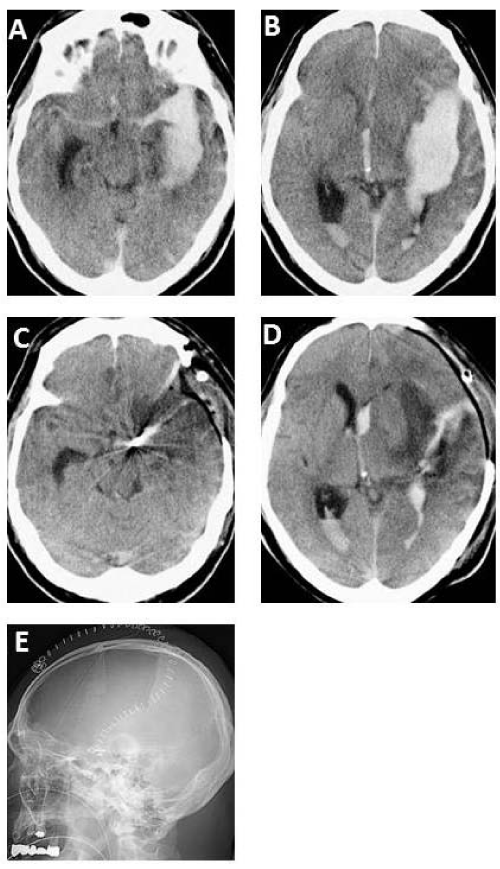
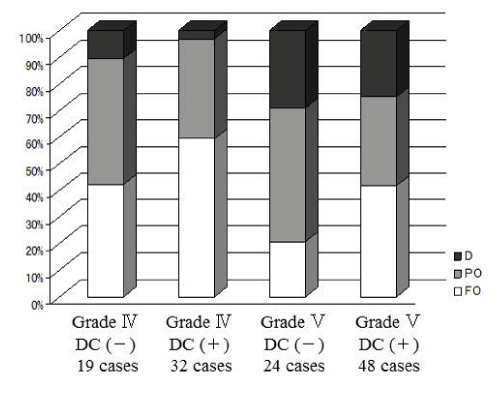
A 57–year–old man suffered sudden onset of headache with loss of consciousness. Computed tomography (CT) scan on admission showing diffuse SAH with ICH (Figure 1A,1B). Three dimensional CT angiogram revealing a large aneurysm at the internal cerebral artery (ICA) bifurcation. The patient underwent aneurysm clipping with primary DC (Figure 1E). Two days after the operation, the brain edema had progressively worsened, on the other hand, midline shift and brain stem compression improved (Figure 1C, 1D). The patient recovered with Glasgow Outcome Scale score of Moderate disability(MD). In addition, one hundred and twenty three consecutive patients with poor–grade SAH (grades IV and V) were treated in our hospital. We retrospectively reviewed the hospital records including medical charts, operative records, and radiological findings. Thus, we divided the clinical outcomes of the patients into four groups with or without DC in grade IV and V. Patient outcome was assessed on discharge using Glasgow Outcome Scale that comprises five levels: good recovery (GR), moderate disability (MD), severe disability (SD), vegetative state (VS), and death (D). The clinical findings summarized in Table 1, which showed that favorable outcome increased after DC (Figure 2).
Grade IV
Grade V
DC (-)
DC (+)
DC (-)
DC (+)
Patient number
19
32
24
48
Age
37-77
35-72
35-75
30-74
mean
59.4
56.6
57.8
56.8
Gender
Male
5
13
14
19
Female
14
19
10
29
Site
R
11
20
12
21
L
8
12
12
27
Aneurysm size
Small
17
28
20
42
Medium
2
2
2
3
Large
0
2
2
3
Re-rupture
1
5
3
7
Intraoperative rupture
1
5
3
5
Hypothermia therapy
0
4
2
12
Aneurysm site
ICA
4
7
5
13
MCA
13
23
10
32
AcomA
2
2
9
3
Hematoma volume (mm3)
38.8
39.6
39.3
41.5
Midline shift (mm)
4.33
7.81
6.28
8.5
Basal cistern effacement
Yes
2
3
2
3
No
17
29
22
45
Brain swelling
3
13
16
35
DC: Decompressive Craniectomy; ICA: Internal Carotid Artery; MCA: Middle Cerebral Artery; AcomA: Anterior Communicating Artery
Table 1: Clinical characteristics of 123 patients who treated in poor-grade SAH.
Figure 1: A 57-year-old man suffered sudden onset of headache with loss of consciousness. CT scan on admission (A, B) showing diffuse subarachnoid hemorrhage (SAH) with intracerebral hematoma. Three dimensional CT angiogram revealing a large aneurysm at the internal cerebral artery bifurcation. The patient underwent aneurysm clipping with primary decompressive craniectomy (E). Two days after the operation, the brain edema had progressively worsened, on the other hand, midline shift and brain stem compression improved (C, D). The patient recovered with Glasgow Outcome Scale score of Moderate disability (MD).
Figure 2: Clinical outcomes for poor-grade SAH patients (grades IV and V) divided into four groups with or without DC in our hosipital, showing that favorable outcome increased after DC. D: death, PO: Poor Outcome, FO: Favorable Outcome.
Discussion
Surgical outcome in poor–grade aneurysmal SAH patients
Previous analysis has revealed that intraoperative aneurysm rupture has no impact on the outcome in patients with good or poor initial condition. Initial clinical grades IV and V as well as initial Fisher grades III and IV were strongly associated with poor outcome [13]. In addition, the presence of intracerebral or intraventricular hemorrhage has been identified as one of the common factors to influence the outcome [14,15]. Early aggressive surgery and intensive care for SAH patients in poor clinical condition can improve the mortality and morbidity [16–18]. Despite recent advances in the treatment of poor–grade SAH, the surgical outcome continues to be unfavorable in many patients because of uncontrollable intracranial hypertension. Increased ICP is associated with several detrimental effects such as cerebral ischemia following reduced perfusion pressure [19]. Intractable intracranial hypertension is associated with poor outcome among patients with poor–grade aneurysmal SAH [20– 23]. Therefore, immediate and continued effective ICP management is associated with improved outcome [16].
Pathophysiology of poor–grade SAH with hematoma
The clinical outcome for SAH patients with ICH is usually worse than that for SAH patients without ICH [16,24,25]. ICP increased in over half of all patients with SAH, particularly among those with poorgradeSAH and ICH [26]. On the other hand, the clinical outcome for SAH patients with ICH does not significantly differ from that of SAH patients without associated hematoma [27]. The incidence of rebleeding in patients with poor–grade SAH is 22% with ICH and 14% without ICH. Clinical grades on admission were significantly higher and outcome at 6 months after onset was less favorable in patients with poor–grade SAH with ICH than in those without ICH. Larger ICH was associated with worse clinical grade and less favorable outcome. The sites of the hematoma and ruptured aneurysm were closely correlated. The poor outcome of patients with ICH seems to be related to the severity of clinical grade on admission. However,the two groups did not significantly differ in terms of managementand surgical outcome for the same clinical grades [28]. Initial brain damage caused by ICH and subsequent brain edema seemed to be the main cause of disability, and might be related to the poor clinical outcome. Intracranial hypertension in patients with SAH can be associated with deleterious changes, which might have profound impacts on outcome [29]. Consequently, aggressive clot evacuation and aneurysm obliteration are recommended. Rapid control of ICP has been associated with improved outcome, indicating that early control of ICP and cerebral blood flow is important to improve outcome or to counteract deterioration. The management of SAH patients depends on the prevention of secondary brain damage. High ICP within the fixed volume of the skull can lead to secondary brain damage, herniation, and permanent neurological damage, or even death. Severe malignant vasospasm may develop and become resistant to conventional medical treatments in some patients. For these reasons, new additional therapeutic strategies such as DC are essential to improve the clinical outcome in patients with poor–grade SAH.
Efficacy and pathophysiology of DC
DC is a neurosurgical procedure which is intended to treat high ICP. DC includes removal of the calvarial bones to create free space which the brain can occupy under the scalp, aiming to minimize ischemic damage by increasing cerebral blood flow [30–33]. Recent clinical findings suggest that external decompression might reduce ICP [34–37]. Craniectomy and enlarged dural plasty induce a significant decrease in ICP and increase in cerebral tissue oxygenation [35,36]. External decompression may also significantly increase cerebral tissue oxygenation [34,37]. In addition, clinical studies have suggested that DC results in significant elevation of mean cerebral blood flow velocity and significant decreases in the MCA pulsatility index values, indicating reduction in cerebrovascular resistance in most patients with traumatic brain swelling [24,38]. Recent studies suggested that DC with dural augmentation enlarges the intracranial space so that the swollen cerebral hemisphere could expand out of the normal cranial limits, thus avoiding progression of brain herniation. The gain in intracranial volume results in both improvement of cerebral compliance and decrease in ICP; the latter favors increases in both cerebral blood flow and cerebral microvascular perfusion, which can be accompanied by higher brain tissue oxygen tension as well as the normalization of abnormal metabolic parameters in patients with cerebral ischemia [7–9]. Therefore, DC can induce immediate reduction in ICP and control the ICP elevation that occurs several days after SAH. Surgical outcome improves in cases with controlled ICP by DC, compared with cases with uncontrolled ICP [26,27]. In particular, delayed swelling can induce elevated ICP, which can be confused with the symptoms of vasospasm. Therefore, early DC would be useful to avoid this clinical problem.
Surgical outcome of DC for SAH
Recent clinical studies have found that DC can improve the surgical outcome of patients with poor–grade SAH and massive ICH [10–12]. The surgical outcome was improved by ICP control in a series of 8 patients with poor–grade MCA aneurysmal SAH and sylvian hematoma treated with external decompression, resulting in a favorable outcome rate of 62.5% compared with a poor outcome rate of 37.5% [12]. External decompression resulted in favorable outcome rates of 33% in grade IV and 40% in grade V patients with sylvian hematoma [11]. Long–term outcome was better for patients who underwent secondary DC within the first 48 hours after SAH [39]. DC can be a life–saving procedure which provides a better outcome in patients with cerebral infarction secondary to vasospasm and SAH [40]. DC was most beneficial if performed immediately after the detection of resistant increase in ICP in patients younger than 60 years old, suggesting that early intervention may have a great influence on the outcome [40]. Although DC has prolonged the short–term survival of patients with poor–grade SAH and ICH, the overall quality of life experienced by survivors remains poor [10]. Further investigation is required to clarify long–term outcomes with particular focus on higher cerebral function.
DC for SAH with ASDH
The incidence of aneurysmal SAH associated with ASDH is between 0.5% and 7.9% [24,41] and leads to a poor clinical outcome [42,43]. SAH patients with ASDH have a poorer prognosis compared to SAH patients without ASDH [44]. The poor outcome in SAH patients with ASDH seems to be related to the severity of the clinical grade on admission. Initial brain damage caused by ASDH, and the subsequent brain edema, seem to be the main causes of disability, which is closely related to the poor clinical outcome. Therefore, aggressive clot evacuation and aneurysm obliteration are recommended for rapid control of the ICP, which is associated with improved outcome. Early control of the ICP and cerebral blood flow is important for improving the outcome and to prevent deterioration. In general, patients with aneurysmal SAH and ASDH tend to have a worse prognosis compared to SAH patients without ASDH [20]. If patients with aneurysmal ASDH suffer no primary damage to the brain tissue, the prognosis seems to be more favorable if adequate diagnostic investigations and prompt aggressive treatment are performed. The poor outcome in patients with ASDH seems to be due to the initial elevated ICP caused by the ASDH. Despite recent advances in the treatment of patients with poor–grade SAH, surgical outcome remains generally poor because of the uncontrolled intracranial hypertension. Elevated ICP has been associated with several detrimental effects, such as cerebral ischemia and reduction of the cerebral blood flow [20]. In previous series, the mortality rate was 21% and the poor prognosis rate was 42% in patients with aneurysmal SAH and ASDH [45]. Favorable outcome was achieved in 41.7% of SAH patients with ASDH who underwent primary DC [46]. Therefore, rapid DC will be useful for controlling the ICP, thus improving the outcome and decreasing the mortality rates in such patients.
DC for SAH with massive hematoma
Recent clinical studies have reported that DC can be useful to improve the surgical outcome in patients with poor–grade SAH and massive ICH [10,11,34,36]. Surgical outcome was improved by ICP control in a small series of 8 patients with poor–grade MCA aneurysmal SAH and sylvian hematoma treated with external decompression [12]. Favorable outcome was achieved in 5 of the 8 patients and poor outcome in 3 patients. Favorable outcome rates were 33% in grade V patients and 40% in grade IV patients with sylvian hematoma treated with external decompression [11]. Similarly, favorable outcome was obtained in 56.0% of grade IV patients after treatment which included decompression [47]. The ICP remained below 20 mmHg for 7 days after surgery in all patients with favorable outcome, but tended to remain at or over 20 mmHg after surgery in the patients with poor outcome. In addition, the ICP remained over 25 mmHg in the patients who died. Favorable outcomes were found in 52.6% of cases after 12 months [48]. Similarly, good outcome was obtained in 37.5% of patients compared to overall 26.6% in all craniectomy patients at 6 months [49]. These findings suggested that patients with progressive cerebral edema may benefit most from secondary DC after SAH. DC prolonged the short–term survival of patients with poor–grade SAH with ICH, but the overall quality of life for survivors was still poor. Increased ICP refractory to standard treatment in a patient with SAH can lead to poor outcome and mortality. Several studies found that DC significantly improves outcome due to increased perfusion pressure or reduced ICP. We suggest that DC helps to reduce morbidity and mortality through control of elevated ICP in patients with poor–grade SAH. However, further investigation is required to clarify the value of DC for treating poor–grade SAH from the viewpoint of long–term higher cerebral function.
References
- Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003; 60: 227-232.
- Kondziolka D, Fazl M. Functional recovery after decompressive craniectomy for cerebral infarction. Neurosurgery. 1988; 23: 143-147.
- Mori K, Aoki A, Yamamoto T, Horinaka N, Maeda M. Aggressive decompressive surgery in patients with massive hemispheric embolic cerebral infarction associated with severe brain swelling. Acta Neurochir (Wien). 2001; 143: 483-491.
- Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998; 29: 1888-1893.
- Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006; 104: 469-479.
- Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997; 41: 84-92.
- Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, de Oliveira MM, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg. 2012; 117: 589-596.
- Bor-Seng-Shu E, Figueiredo EG, Fonoff ET, Fujimoto Y, Panerai RB, Teixeira MJ, et al. Decompressive craniectomy and head injury: brain morphometry, ICP, cerebral hemodynamics, cerebral microvascular reactivity, and neurochemistry. Neurosurg Rev. 2013; 36: 361-370.
- Lazaridis C, Czosnyka M. Cerebral blood flow, brain tissue oxygen, and metabolic effects of decompressive craniectomy. Neurocrit Care. 2012; 16: 478-484.
- D'Ambrosio AL, Sughrue ME, Yorgason JG, Mocco JD, Kreiter KT, Mayer SA, et al. Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: clinical outcome and quality of life assessment. Neurosurgery. 2005; 56: 12-19.
- Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997; 87: 170-175.
- Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery. 2002; 51: 117-124.
- Sandalcioglu IE, Schoch B, Regel JP, Wanke I, Gasser T, Forsting M, et al. Does intraoperative aneurysm rupture influence outcome? Analysis of 169 patients. Clin Neurol Neurosurg. 2004; 106: 88-92.
- Rosen DS, Macdonald RL. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005; 2: 110-118.
- Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007; 38: 2315-2321.
- Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990; 72: 559-566.
- Brandt L, Sonesson B, Ljunggren B, Säveland H. Ruptured middle cerebral artery aneurysm with intracerebral hemorrhage in younger patients appearing moribund: emergency operation? Neurosurgery. 1987; 20: 925-929.
- Seifert V, Trost HA, Stolke D. Management morbidity and mortality in grade IV and V patients with aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 1990; 103: 5-10.
- Hayashi M, Kobayashi H, Kawano H, Yamamoto S, Maeda T. Cerebral blood flow and ICP patterns in patients with communicating hydrocephalus after aneurysm rupture. J Neurosurg. 1984; 61: 30-36.
- Kaye AH, Brownbill D. Postoperative intracranial pressure in patients operated on for cerebral aneurysms following subarachnoid hemorrhage. J Neurosurg. 1981; 54: 726-732.
- Le Roux PD, Elliott JP, Downey L, Newell DW, Grady MS, Mayberg MR, et al. Improved outcome after rupture of anterior circulation aneurysms: a retrospective 10-year review of 224 good-grade patients. J Neurosurg. 1995; 83: 394-402.
- Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed cases. J Neurosurg. 1996; 85: 39-49.
- Takeuchi S, Koike T, Sasaki O, Kamada K, Tanaka R, Arai H, et al. Intracranial extradural pressure monitoring after direct operation on ruptured cerebral aneurysms. Neurosurgery. 1989; 24: 878-883.
- Barton E, Tudor J. Subdural haematoma in association with intracranial aneurysm. Neuroradiology. 1982; 23: 157-160.
- Hauerberg J, Eskesen V, Rosenørn J. The prognostic significance of intracerebral haematoma as shown on CT scanning after aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1994; 8: 333-339.
- Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004; 101: 408-416.
- Abbed KM, Ogilvy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. 2003; 15: E4.
- Tokuda Y, Inagawa T, Katoh Y, Kumano K, Ohbayashi N, Yoshioka H. Intracerebral hematoma in patients with ruptured cerebral aneurysms. Surg Neurol. 1995; 43: 272-277.
- Yoshimoto Y, Wakai S, Satoh A, Hirose Y. Intraparenchymal and intrasylvian haematomas secondary to ruptured middle cerebral artery aneurysms: prognostic factors and therapeutic considerations. Br J Neurosurg. 1999; 13: 18-24.
- Akyuz M, Ucar T, Acikbas C, Kazan S, Yilmaz M, Tuncer R, et al. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg. 2010; 20: 382-389.
- Kakar V, Nagaria J, John Kirkpatrick P. The current status of decompressive craniectomy. Br J Neurosurg. 2009; 23: 147-157.
- Kilincer C, Asil T, Utku U, Hamamcioglu MK, Turgut N, Hicdonmez T, et al. Factors affecting the outcome of decompressive craniectomy for large hemispheric infarctions: a prospective cohort study. Acta Neurochir (Wien). 2005; 147: 587-594.
- Ucar T, Akyuz M, Kazan S, Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005; 22: 1311-1318.
- Jaeger M, Soehle M, Meixensberger J. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003; 74: 513-515.
- Jourdan C, Convert J, Mottolese C, Bachour E, Gharbi S, Artru F, et al. [Evaluation of the clinical benefit of decompression hemicraniectomy in intracranial hypertension not controlled by medical treatment]. Neurochirurgie. 1993; 39: 304-310.
- Reithmeier T, Löhr M, Pakos P, Ketter G, Ernestus RI. Relevance of ICP and ptiO2 for indication and timing of decompressive craniectomy in patients with malignant brain edema. Acta Neurochir (Wien). 2005; 147: 947-951.
- Stiefel MF, Heuer GG, Smith MJ, Bloom S, Maloney-Wilensky E, Gracias VH, et al. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004; 101: 241-247.
- Bor-Seng-Shu E, Hirsch R, Teixeira MJ, De Andrade AF, Marino R Jr. Cerebral hemodynamic changes gauged by transcranial Doppler ultrasonography in patients with posttraumatic brain swelling treated by surgical decompression. J Neurosurg. 2006; 104: 93-100.
- Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 2007; 38: 987-992.
- Tuzgen S, Kucukyuruk B, Aydin S, Ozlen F, Kizilkilic O, Abuzayed B, et al. Decompressive craniectomy in patients with cerebral infarction due to malignant vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosci Rural Pract. 2012; 3: 251-255.
- Bassett RC, Lemmen LJ. Subdural hematoma associated with bleeding intracranial aneurysm. J Neurosurg. 1952; 9: 443-450.
- Kamiya K, Inagawa T, Yamamoto M, Monden S. Subdural hematoma due to ruptured intracranial aneurysm. Neurol Med Chir (Tokyo). 1991; 31: 82-86.
- Weir B, Myles T, Kahn M, Maroun F, Malloy D, Benoit B, et al. Management of acute subdural hematomas from aneurysmal rupture. Can J Neurol Sci. 1984; 11: 371-376.
- Ohkuma H, Shimamura N, Fujita S, Suzuki S. Acute subdural hematoma caused by aneurysmal rupture: incidence and clinical features. Cerebrovasc Dis. 2003; 16: 171-173.
- Koerbel A, Ernemann U, Freudenstein D. Acute subdural haematoma without subarachnoid haemorrhage caused by rupture of an internal carotid artery bifurcation aneurysm: case report and review of literature. Br J Radiol. 2005; 78: 646-650.
- Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Clinical characteristics and surgical outcomes of patients with aneurysmal subarachnoid hemorrhage and acute subdural hematoma undergoing decompressive craniectomy. World Neurosurg. 2011; 75: 73-77.
- Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis. 2008; 26: 612-617.
- Buschmann U, Yonekawa Y, Fortunati M, Cesnulis E, Keller E. Decompressive hemicraniectomy in patients with subarachnoid hemorrhage and intractable intracranial hypertension. Acta Neurochir (Wien). 2007; 149: 59-65.
- Güresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus. 2009; 26: E4.