Research Article
Austin Neurosurg Open Access. 2014;1(2): 1009.
Classification of Headaches Associated with Rathke's Cleft Cyst According to Their Onset and Duration: A Clinical Study
Yasuhiko Hayashi1*, Daisuke Kita1, Masayuki Iwato1, Issei Fukui1, Hiroki Sano1, Yutaka Hayashi1, Osamu Tachibana2 and Jun-ichiro Hamada1
1Department of Neurosurgery, Kanazawa University, Japan
2Department of Neurosurgery, Kanazawa Medical University, Japan
*Corresponding author: Yasuhiko Hayashi, Department of Neurosurgery, Graduate School of Medical Science, Kanazawa University, Address; 13-1, Takaramachi, Kanazawa, 920-8641, Japan
Received: March 05, 2014; Accepted: May 07, 2014; Published: May 08, 2014
Abstract
Objective: Although Rathke cleft cysts (RCCs) are typically symptomatic, headaches can be one of the common symptoms, together with visual impairment and endocrinological abnormalities. Although several factors affecting the development of headache have been reported, the detailed mechanism are still unknown.
Methods: This retrospective study included 62 patient with RCCs whose symptom was headache and who were examined with using of magnetic resonance (MR) imaging, intraoperative findings, and pathology of surgical specimens.
Results: The characteristics of headache onset and duration were divided into three types. Apoplexy type headaches revealed hyper intensity on T1– weighted images (WI) and hypointensity on T2–WI of MRI. All patients with apoplexy characteristically had waxy nodules in their cysts, which showed as extreme hypointensities on T2–WI. Acute type headaches revealed hyper intensity on T1–WI with inconsistent intense signals on T2–WI. Chronic type headaches showed hypointensity on T1–WI in 12 patients (50%) and hyper intensity on T2–WI, similar to cerebrospinal fluid, and hyper intensity on T1–WI, with hyper intensity in 5 and hypointensity in 7 on T2–WI in the other 12 (50%). Cyst size was significantly large in chronic type. Pathological examination demonstrated that intracystic hemorrhage was dominant in apoplectic type. In chronic type headache, both infiltration of collagen tissue and inflammatory cells were characteristically detected.
Conclusion: In acute and apoplectic types, inflammation spread by cyst contents to the surrounding structures was suspected. Intracystic hemorrhage was also suggested as possible causes, particularly for apoplectic type headache. With respect to chronic type, two different mechanisms, mechanical compression and continuous infiltration of inflammatory cells were considered.
Keywords : Rathke cleft cyst; Headache; Magnetic resonance imaging; Pathology
Abbreviations
CSF: Cerebrospinal Fluid; MR: Magnetic Resonance; RCC: Rathke Cleft Cyst
Introduction
In recent years, using modern neuroimaging, Rathke cleft cysts (RCCs) have been detected as small, asymptomatic sellar lesions [1,2]. Although their incidence in normal autopsy reports is high (13 to 22%), RCCs rarely become symptomatic throughout life [3– 5]. As a result of the recognition of RCCs as distinct clinical entities, many studies have been conducted on the neuroradiological and pathological features of RCCs [2,6,7].
In many patients, RCCs are still detected incidentally without obvious clinical symptoms; on the other hand, RCCs occasionally manifest as characteristic symptoms, such as headache, visual impairment, and endocrinological abnormalities [2,7–9]. Although many authors have reported details about the identification of differential neuroimaging features of intra– and supra–sellar cystic mass lesions including RCC, there are still few reports about thecorrelation between clinical symptoms, neuroimaging features, and characteristics of cyst contents of RCCs [6,9–11]. Although it is apparent that it is clinically meaningful to compare and correlate the signal intensities of magnetic resonance (MR) images with the contents of RCCs, there are many exceptions to the correlations that do not follow the trends reported [12,13].
Headaches are well known to be a common presentation of RCCs and manifest in 44 to 59% of symptomatic patients with RCCs [12,14]. As headaches associated with RCCs have not been well characterized, they are not described in the International Classification of Headache Disorders, 2nd edition (ICHD–II) [14]. Moreover, there are few studies about the characteristics and underlying mechanisms that cause headache in the patients with RCCs. Thus, we wanted to investigate which factors lead to major clinical symptoms of RCCs by conducting a retrospective review of the neuroimaging and pathological studies of RCCs in our institute.
Clinical Materials and Methods
This retrospective study involved 91 patients presenting with RCCs in our institute between 1994 and 2012. Among them, 62 patients (68%) presented with headaches and other 29 patients (32%) presented with other symptoms or found incidentally. Among the 62 patients whose symptoms were headache, operations were performed in 43 patients and postoperative histological results were confirmed as RCC in 39 patients. All patients underwent MRI, and visual and endocrinological examinations. RCC size was evaluated using MRI, with or without gadolinium–enhanced T1–weighted images (WIs) on axial, coronal, and sagittal sections. The signal intensities of cystic contents on T1–and T2–WIs and the presence of waxy nodules (a non–enhanced intracystic nodule whose signals are hyper–intense on T1 and very hypo–intense on T2) were assessed.
A preoperative diagnosis of RCCs was made on the basis of the following MRI findings that are characteristic of RCCs; cystic lesions in the sellar or suprasellar region, absence or faint cystic wall enhancement, normal pituitary gland around the cyst, and absence of calcification. Moreover, intraoperative findings comparable to RCCs were considered when the cysts' contents were white or yellow, with varying viscosities of mutinous liquid and without any nodule of calcification. Postoperatively, final diagnosis was made using surgical specimens from 39 patients, with a light microscopic analysis demonstrating the pathological findings as follows; the epithelium lining consisted of the cyst wall with cubical or columnar cells, cilia on the cell surface inside the cyst, and goblet cells. Four patients were diagnosed using MRI and intraoperative findings only because of a lack of surgical specimen. Another 19 patients without surgical treatment were diagnosed by MRI findings only.
Headaches were divided into three types according to their onset and duration; apoplectic, headache with sudden onset manifesting like a pituitary apoplexy; acute, unusual episodic headache occurred within one month before consultation and patients remembered the onset clearly; and, chronic, headache persisted for more than a month and patients did not remember the onset clearly. The location and duration of headache was obtained from the patient records.
Chi–square and Post–hoc tests were used for comparison between two groups out of three. Chi–square test (two–tailed) was applied for when analyzing sex difference, T1–WI and T2–WI signals, the presence of endocrinological abnormalities, and pathological observations. Post–hoc test was applied when analyzing patients' ages and size of cysts. A p–value of <0.05 was considered statistically significant.
Results
In our study, headache was the most common manifestation and occurred in 62 patients (68%). This cohort consisted of 17 males and 45 females, with an age at diagnosis ranging from 10 to 67 years (mean 34.2). Among these patients with headache, 11 (18%) presented with visual acuity and field disturbance, and 30 (48%) with endocrinological abnormalities. Meanwhile, 29 patients were diagnosed with RCC with symptoms other than headache. This cohort consisted of 11 males and 18 females, with an age range of 6 to 74 years (mean 40.9). In these patients, visual impairment was identified in 11 (38%), endocrinological abnormalities were found in 21 (72%), and other symptoms in 6 patients (21%) (Table 1).
Apoplectic type headaches were found in 15 patients (24%), 1male and 14 females, with an average age of 31.7 years. Their MRI findings were uniform in content, hyper intense on T1–WI and hypo intense on T2–WI. The average of maximum diameters in apoplectic type of RCCs was 15.3 mm. In particular, characteristic waxy nodules, showing marked hypointensity on T2–WI, were seen in all 15 patients (100%) (Table 1).
Acute type headaches were found in 23 patients (37%), 9 males and 14 females, with in average age of 34.3 years. Their MRI findings were hyper intense in uniform on T1–WI; however, hypo intense in 18 patients and hyper intense in 5 on T2–WI were found. Signal intensities of their cyst contents were regular in 14 patients and irregular in 9. The average of maximum diameters of this type was 14.6 mm, which was not statistically significant compared to cysts detected in apoplectic type (p=0.708). Waxy nodules were detected in 8 patients (35%) (Tables 1,3).
Chronic type headaches were found in 24 patients (39%), 7 males and 17 females, with an average age of 35.4 years. The MRI findings of their cyst contents could be divides into two groups. The first group (12 patients) showed hypointensity on T1–WI and hyper intensity on T2–WI, which was almost identical as cerebrospinal fluid (CSF). Intraoperatively, the cyst content of this group was observed as a clear fluid similar to CSF. The second group (also 12 patients) showed hyper intensity on T1–WI, and hypointensity, or hyper intensity on T2–WI, in 7 and 5 patients, respectively. Intraoperatively, the cyst content of this group was observed as a mutinous fluid with hyper viscosity. Signal intensities of their cyst contents were regular in 14 patients and irregular in 10. The average of maximum diameters of RCCs in this type was 19.5 mm, which was significantly larger than that from the other two types (p=0.0014 to apoplectic type and p<0.0001 to acute type, Post–hoc test). But the average of maximum diameters of RCCs between two groups of this type was not statistically significant (p=0.581, Post–hoc test). Waxy nodules were found only in 2 patients (8%) (Tables 1,3).
The location of the headaches was primarily in the frontal region (41 patients, 66%) regardless of the type of headache. Pain recognition in the retrobulbar region was detected and took a second place by 11 patients (18%), and following as occipital region in 5 (8%), temporalregion in 4 (6%), and parietal region in one (2%) (Tables 1,3).
Across all the patients, RCCs that were hypo intense on T1–WI and hyper intense on T2–WI (n = 12) had an average of maximum diameters of 20.6 mm in this type and fluid similar in appearance to CSF in the cysts, while RCCs that were hyper intense on T1–WI (n= 50) had an average of maximum cyst diameter of 15.3 mm and white⁄ yellow–colored, hyper viscous mutinous fluid within the cysts. The difference between the maximum cyst diameters of these two groups was statistically significant (p= 0.0008, Post–hoc test) (Tables 1,3).
Surgery was performed with procedures, such as aspiration of cyst content and partial resection of the cyst wall, in 43 out of 62 patients experiencing headaches. Among the 43 patients, 39 underwent transsphenoidal surgery and 4 underwent transcranial surgery. Of the patients who underwent transsphenoidal surgery, a microscope was used for 26 patients from 1994 to 2005, and an endoscope was used for 16 patients from 2006 to 2012. All transcranial surgeried were performed via the standard pterional approach (Table 1).
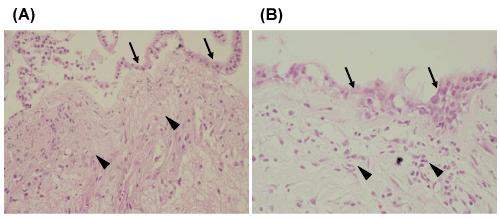
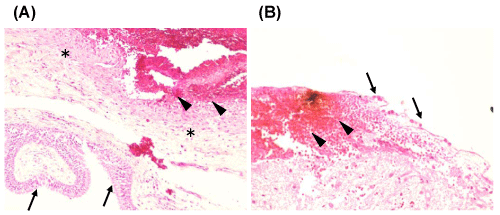
Pathological confirmation was obtained in 39 patients, and microscopic analysis was performed to assess the infiltration of collagen fibers, invasion of inflammatory cells (Figure 1), and intracystic hemorrhages (Figure 2). In apoplectic type headaches (surgical specimens from 10 patients), invasion of inflammatory cells was detected in 9 (90%), and intracystic hemorrhage in 6 (60%), whereas proliferation of collagen tissue was only found in 2 (20%). In all patients with acute type headache (surgical specimens obtained from 12 patients), invasion of inflammatory cells, and proliferation of collagen tissue was found in 6 cases (50%) and intracystic hemorrhage in only 2 (17%). In chronic type headache (surgical specimens from 17 patients), proliferation of collagen tissue was observed in 16 (94%) and invasion of inflammatory cells in 10 (59%), whereas, intracystic hemorrhage was observed in only in 1 (6%) (Tables 2,3). Characteristic observations of intracystic hemorrhage were found in intracystic granulation tissue beneath the epithelial cells (Figure 2).
Figure 1: Photomicrographs of typical histological staining of (A) infiltration of collagen tissue (arrowheads) just beneath the columnar epithelium (arrows) and (B) invasion of inflammatory cells (arrowheads) under the cyst wall (arrows). Hematoxylin & Eosin; original magnification x 100 (A), x 200 (B).
Figure 2: Typical histological pictures of intracystic hemorrhage showing (A) bleeding (arrowheads) from intra cystic granulation tissue (asterisks) beneath the columnar epithelial cells (arrows) and (B) bleeding (arrowheads) directly under the cuboidal epithelial cells (arrows). Hematoxylin & Eosin; original magnification x 100 (A), x 200 (B).
The functional outcomes on headaches were as follows; 39 patients (91%) reported the postoperative disappearance of their headache, while 3 patients (2 chronic and 1 acute, 9%) reported persistent headaches postoperatively. Three patients (one of each type) experienced a recurrence of headache; two of the three (acute and chronic) were associated with relapse of the cyst. Reoperation was performed with transsphenoidal surgery in both patients, resulting in the postoperative relief of headache. The other four patients who persisted headache after 1st or 2nd surgery were treated with medication if necessary (Tables 1,3).
Discussion
The RCCs–associated headaches were divided into three types according to their onset and duration; apoplectic, acute, and chronic. The intensity of apoplectic type revealed hyper on T1–WI and hypoon T2–WI on MRI. That of acute type revealed hyper on T1–WI but inconsistent signals on T2–WI. Chronic type showed hypo on T1–WI and hyper on T2–WI in 50% of patients, and hyper on T1–WI with inconsistent signals on T2–WI, like as acute type, in the other 50%. The average of maximum diameters of cysts was significantly large in chronic type. Pathological examination demonstrated intracystic hemorrhage in apoplectic type, and both collagen tissue infiltration and inflammatory cell invasion were detected in chronic type.
Previous reports have described headaches in patients with RCCs that were frequently non–pulsating, with bilateral, frontal or retroorbital pain, and mimicking those with pituitary adenomas [9,14]. However, mechanisms causing these headaches remained unknown and there were few reports concerning correlations between the headaches and the MRI and pathological findings. In addition, some recent reports suggested patients presenting with sudden onset episodic headache resembled those with pituitary apoplexy, which provided a potential new mechanism for RCC–induced headaches [2,15,16].
Many authors have suggested that the development of headache due to RCCs involved the following mechanisms; compression or displacement of surrounding pain–sensitive structures, such as the diaphragm sellae [17,18]; spread of chemicals from cyst contents inducing inflammation in the adjacent adenohypophysis or neurohypophysis [6,10,19,20]; rupture of cyst wall letting cyst contents into the subarachnoid space [21,22]; and intracystic hemorrhage from the cyst wall [11,14,23]. Hypopituitarism, caused by the spread of inflammatory cyst contents to the hypothesis, also leads to irreversible changes and aggravates headache [14,16,17]. In our report, headaches caused by RCCs were divided into three types according to their onset and duration, and the correlations between MRI findings, pathological examination, and clinical features of headaches were analyzed.
Apoplectic type headaches were characterized as a sudden onset of severe headache, like a pituitary apoplexy. From MRI findings, the signals of the cyst contents revealed hyper intensity on T1–WI and hypointensity on T2–WI in all patients (Table 1). The histological findings demonstrated the invasion of inflammatory cells and bleeding from granulation tissue under the epithelial cell lining in 9 and 6 out of 10 surgical specimens, respectively (Table 2). Thus, our results suggested that intracystic hemorrhage and the spread of cyst contents resulting in inflammatory reactions around surrounding structures are viable potential mechanisms. Other authors have speculated that bleeding from granulation tissue in the cyst wall or bleeding from the portal system of the hypothesis due to the compression of the pituitary stalk, which leads to hypopituitarism, might be another possible mechanism [11,24].
Characters in each type of headache
Type
Number
Gender
Age
MRI (T1)
MRI (T2)
Waxy nodule
E.A.
V.D.
Size (mm)
Location
Duration
Surgery
w/o improvement
Recurrence
(%)
(years)
(%)
(%)
(%)
(%)
(%)
(mm)
after surgery
after surgery
Apoplexy
15 (24)
1 ; 14
31.7
hyper 15 (100)
hypo 15 (100)
15 (100)
9 (60)
2 (13)
15.3
frontal 11 (73)
1-6d
11 (73)
0 (0)
1 (7)
temporal 2 (13)
TC 0, TS 11
0 (0)
0 (0)
occipital 1 (7)
retro-bulber 1 (7)
Acute
23 (37)
9 ; 14
34.3
hyper 23 (100)
hypo 18 (78)
6 (26)
5 (28)
3 (17)
14.6
frontal 16 (70)
1w-1m
14 (61)
1 (4)
1 (4)
hyper 5 (22)
2 (9)
3 (60)
1 (20)
retro-bulber 4 (17)
1m
TC 2, TS 12
1 (100)
1 (100)
occipital 2 (9)
temporal 1 (4)
Chronic
24 (39)
7 ; 17
35.4
hypo 12 (50)
hyper 12 (50)
0 (0)
6 (50)
4 (33)
19.6
frontal 14 (58)
2m-5y
18 (75)
2 (17)
1 (9)
hyper 12 (50)
hyper 5 (21)
1 (4)
7 (58)
1 (8)
retro-bulber 6 (25)
4m-6y
TC 2, TS 16
1 (50)
1 (100)
hypo 7 (29)
1 (4)
occipital 2 (8)
parietal 1 (4)
temporal 1 (4)
Total
62
17:45
34.2
hyper 50 (81)
hyper 37 (60)
25 (40)
30 (48)
11 (18)
16.7
frontal 41 (66)
43 (69)
3 (9)
3 (9)
hypo 12 (19)
hypo 25 (40)
retro-bulber 11 (17)
TC 4, TS 39
2 (66)
2 (66)
occipital 5 (8)
temporal 4 (6)
parietal 1 (2)
Others
29
11:18
40.9
21
11
*
*
22
*
2
TC 2, TS 20
Table 1: Clinical and neuroradiological data in each type of headache.
Acute type headaches were defined as an unusual episodic headache that occurred within a month before consultation, and where patients remembered the onset clearly. The MRI findings revealed hyper intensity on T1–WI in all 23 patients, but hypointensity in 18 patients and hyper intensity in five patients on T2–WI (Table 1). Pathological analysis demonstrated the invasion of inflammatory cells in all patients, but only half of the patients had collagen fiber proliferation, a sign of chronic inflammation (Table 2). Therefore, the spread of inflammatory agents from the cyst was considered as the most likely mechanism.
Table 2: Histological findings in each type of headache.
Statistical Analysis between Each Group (p values)
APO and ACU
APO-CHR
ACU-CHR
sex
0.026**
0.091
0.519
age
0.616
0.469
0.805
MRI-T1
#
0.01*
< 0.0001*
MRI-T2
0.053
0.058
0.044**
size
0.708
0.0014
< 0.0001*
endocrinology
0.126
0.721
0.182
collagen
0.145
< 0.0001*
0.006*
inflammation
0.262
0.087
0.011**
hemorrhage
0.035**
0.0019*
0.882
Table 3: Statistical analysis between each group (p-values).
Chronic type headaches continued for more than a month, and patients did not remember the onset clearly. The MRI findings were roughly divided into two groups. The first group showed signals of cysts that were recognized as similar to CSF, and the size of the cysts in this group (the average of maximum diameters) was statistically larger than the rest of RCCs with hyper intensity on T1–WI. The second group displayed hyper intensity on T1–WI and variable intensities on T2–WI. The cyst content of this group was mutinous fluid (Table 1). However, the average of maximum diameters of RCCs between two groups was not significant. Pathological confirmation revealed massive proliferation of collagen tissue, indicating chronic inflammation and affecting the surrounding structures in all patients except one, and invasion of inflammatory cells in 60% of patients (Table 2). The RCCs with MRI signals of cyst content that were similar to CSF were statistically larger than other RCCs whose signal on T1–WI was hyper intense.
Waxy nodules have been reported in RCCs and are thought to detect in patients with RCC whose symptoms are similar to pituitary apoplexy, but their clinical significance remains unexplored [16,25,26]. Binning et al addressed how the identification of intracystic nodules can aid in the diagnosis of RCC, and histological examinations showed nodules as a mass of cellular debris [25]. Kucharczyk et al reported during pathological examinations that a white nodule, consisting of cellular debris, had adhered to the cyst wall [26]. In our case, however, all patients with RCCs and apoplectic type headaches had nodules. On the other hands, nodules existed in 35% of acute type headaches and in only 8% of chronic type headaches, which are statistically different from the apoplectic type.
We suggest different underlying mechanisms depending on the onset and duration of headaches caused by RCCs. For acute type headaches, inflammation caused by the spread of inflammatory materials was considered. Intracystic hemorrhage was also suggested as one of the causes, especially for apoplectic type headache. Two different mechanisms, mechanical compression of the surrounding structures and chronic spreads of inflammatory reaction were suggested for chronic type headache. In the group of RCCs whose signals on MRI were similar to CSF, mechanical compression leading to stretching stress over the surrounding structures was considered as cause of headache since the size of cysts was statistically larger than other RCCs whose signal on T1–WI was hyper intense.
In our study, frontal headache was the most common presentation in the patients with RCC. This frontal dominancy suggests stimuli to the trigeminal nerve as the final step in causing headache, regardless of mechanical compression or spreading of inflammation [14,16]. The long–term functional outcomes for headaches caused by RCCs were excellent, regardless of the type of headaches. Nishioka et al also reported that frontal episodic headache is a common and characteristic manifestation that is successfully improved after surgery in most patients [14]. Other authors described similar results [7,8,10]. In our study, we encountered only three patients (9%) who underwent surgical aspiration and partial removal of the cyst wall of RCCs who did not experience postoperative relief from headache (Table 1). However, it is interesting that the relapse of headache reported in two patients was associated with a recurrence of RCCs; this result would be compatible even if the causes of headaches were mechanical compression and spreading of inflammation.
Conclusion
In our series, we analyzed the correlation between findings from MRI, pathological examinations, and clinical features of headaches originating from RCCs in order to elucidate a possible mechanism to explain headache development. In our study, the classification of headaches according to onset and duration clearly delineates the different mechanisms that may contribute to the development of each type of headache. RCCs with hypo intense signals on T1–WI manifest as chronic headaches due to compression of the surrounding structures, and RCCs with hyper intense signals on T1–WI manifest as various types of headaches due to the spreading of inflammatory agents, cyst rupture and intracystic hemorrhage.
References
- Greenberg MS. Peripheral nerves. Peripheral neuropathies. Greenberg MS, editors. Handbook of Neurosurgery. 6th edn. New York: Thieme. 2006; 565-569.
- Shapiro BE, Preston DC. Entrapment and compressive neuropathies. Med Clin North Am. 2009; 93: 285-315, vii.
- Wessel LE, Fufa DT, Boyer MI, Calfee RP. Epidemiology of carpal tunnel syndrome in patients with single versus multiple trigger digits. J Hand Surg Am. 2013; 38: 49-55.
- Okamura A, Meirelles LM, Fernandes CH, Raduan Neto J, Dos Santos JB, Faloppa F. Evaluation of patients with carpal tunnel syndrome treated by endoscopic technique. Acta Ortop Bras. 2014; 22: 29-33.
- Bochenek A, Reicher M. Brachial plexus. Median nerve. Lasinski W, editors. In: Human anatomy. 4th edn. Warszawa: Wydawnictwo Lekarskie PZWL. 1998: 51-61.
- Sobotta J. Head, neck, upper limb. Putz R, Pabst R, editors. In: Human anatomy. 1st edn. Wroclaw; Urban and Partner. 1994.
- Demircay E, Civelek E, Cansever T, Kabatas S, Yilmaz C. Anatomic variations of the median nerve in the carpal tunnel: a brief review of the literature. Turk Neurosurg. 2011; 21: 388-396.
- Louis DS, Greene TL, Noellert RC. Complications of carpal tunnel surgery. J Neurosurg. 1985; 62: 352-356.
- Vargas A, Chiapas-Gasca K, Hernandez-Diaz C, Canoso JJ, Saavedra MA, Navarro-Zarza JE, et al. Clinical anatomy of the hand. Reumatol Clin. 2012; 8: 25-32.
- Waloch J, Skawina A, Gorczyca J. Upper limb. Carpal tunnel. Skawina A, editors. In: Human anatomy. Cracow, Jagiellonian University Press. 2002: 65.
- Eversman WW. Entrapment and compression neuropathies. Green DP, editors. Operative hand surgery. 3rd edn. New York: Churchil Livingstone. 1993; 1356-1365.
- Phalen GS. The carpal-tunnel syndrome. Clinical evaluation of 598 hands. Clin Orthop Relat Res. 1972; 83: 29-40.
- Zyluk A, Puchalski P. A comparison of the results of carpal tunnel release in patients in different age groups. Neurol Neurochir Pol. 2013; 47: 241-246.
- Wilbourn AJ, Gilliatt RW. Double-crush syndrome: a critical analysis. Neurology. 1997; 49: 21-29.
- Agee JM, McCarroll HR Jr, Tortosa RD, Berry DA, Szabo RM, Peimer CA. Endoscopic release of the carpal tunnel: a randomized prospective multicenter study. J Hand Surg Am. 1992; 17: 987-995.
- Chow JC. Endoscopic release of the carpal ligament for carpal tunnel syndrome: 22-month clinical result. Arthroscopy. 1990; 6: 288-296.
- Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release : a prospective, randomized trial. J Bone Joint Surg Am. 2002; 84-84A: 1107-15.
- Ferdinand RD, MacLean JG. Endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome. A prospective, randomised, blinded assessment. J Bone Joint Surg Br. 2002; 84: 375-379.
- Brief R, Brief LP. Endoscopic carpal tunnel release: report of 146 cases. Mt Sinai J Med. 2000; 67: 274-277.