Case Report
Austin Neurosurg Open Access. 2014;1(3): 1013.
Oculomotor Nerve Schwannoma Presenting as an Entirely Cystic Homogeneous Mass on Magnetic Resonance Imaging: Case Report
Suenaga J*, Tateishi K, Takase H, Kanno H and Kawahara N
Department of Neurosurgery, Yokohama City University Graduate School of Medicine, Japan
*Corresponding author: Suenaga J, Department of Neurosurgery, Yokohama City University Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-0004, Japan
Received: April 29, 2014; Accepted: June 05, 2014; Published: June 09, 2014
Abstract
A 79–year–old woman presented with left oculomotor nerve paresis. Initial Computed Tomography (CT) demonstrated an isodense cystic lesion 15 mm in diameter in the left crural and carotid cisterns. On Magnetic Resonance Imaging (MRI), the cyst was homogeneously hyperintense on both T1– and T2–weighted imaging with slight hemorrhage. Removal of the lesion was indicated, since the cyst was gradually enlarging and symptoms were progressing. Since the cyst was tightly attached to the oculomotor nerve, partial resection was performed. Pathology of the cyst wall revealed schwannoma with microhemorrhage. However, the residual tumor showed sudden bleeding 1 month later, so second surgery was performed to remove the tumor subtotally, leaving a small piece of residual capsule tightly adhering to the brainstem and internal carotid artery. Although oculomotor nerve schwannoma is rare, particularly presenting as a completely cystic mass, this diagnosis should be considered with such lesions. In addition, total or subtotal resection to prevent further enlargement or hemorrhage might be indicated, even in older patients.
Keywords: Oculomotor nerve schwannoma; Cyst formation; Intratumoral hemorrhage
Abbreviations
CT: Computed Tomography; MRI: Magnetic Resonance Imaging; DWI: Diffusion–Weighted Imaging; VS: Vestibular Schwannoma; CSF: Cerebrospinal Fluid; EMA: Epithelial Membrane Antigen; GKR: Gamma Knife Radiosurgery; FLAIR: Fluid–Attenuated Inversion– Recovery
Introduction
Oculomotor nerve schwannoma in the absence of neurofibromatosis is extremely rare, with only 56 cases previously reported in the literature [1–8]. According to a systematic review by Furtado et al. [2], the most common site of origin is in thecisternal segment of the oculomotor nerve. Although radiological diagnosis is usually made by Magnetic Resonance Imaging (MRI), precise diagnosis is often difficult, and differentiation from other tumors, including meningioma, dermoid cyst, craniopharyngioma,neurenteric cyst, and pituitary adenoma, is warranted. We recently treated a patient with an entirely cystic schwannoma originating from the oculomotor nerve in the crural cistern, which showed rapid growth and intratumoral hemorrhage. Such cystic tumors are extremely rare, and only two cases have been reported [5,6]. We report this case and review the pertinent literature, with particular focus on the radiological features.
Case Presentation
A 79–year–old woman presented to our clinic with left oculomotor nerve paresis. No special past or family history such as neurofibromatosis was noted. Computed Tomography (CT) showed an isodense cystic lesion 15 mm in diameter in the left crural cistern (Figure 1A). Tiny high–density niveau in this mass suggested hemorrhage in the cyst. No calcification or vessel anomaly was seen on CT angiography. MRI also revealed a homogeneous cystic mass, hyperintense on both T1– and T2–weighted imaging (Figure 1B, C). The tumor capsule did not show clear contrast enhancement. The mass was isointense to brainstem on Diffusion–weighted Imaging (DWI). Due to the advanced age of the patient, we continued close observation; however, the cyst gradually enlarged and reached 22 mm in diameter after 6 months (Figure 2). The mass effect of the tumor on the left cerebral peduncle was increased, and oculomotor function gradually deteriorated. We finally decided to resect the tumor inorder to decompress the brainstem, based on a tentative preoperative diagnosis of neurenteric cyst.
Figure 1: A) Axial unenhanced CT at presentation, showing a cystic lesion in the left crural cistern that is is dense to the cisternal space. B, C) Axial unenhanced MRI showing cystic and homogeneous mass. T1-weighted imaging (B) shows a hyperintense mass. T2-weighted imaging (C) shows extreme hyperintensity compared to brainstem. Tiny high-intensity niveau in this mass suggest hemorrhage in the cyst.
Figure 2: Seven months after initial onset, axial unenhanced MRI shows enlargement of the cyst. T1-weighted imaging (left) and T2-weighted imaging (right) also show homogeneous signal intensity and tumor size reaching 22 mm in diameter during this period.
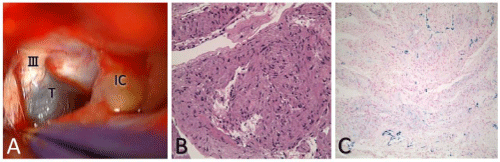
Through a left transsylvian approach, a cystic mass with grayish capsule was observed lateral to the internal carotid artery in the carotid cistern. The cyst was attached to the oculomotor nerve, suggesting a neural origin (Figure 3A). To preserve oculomotor function, partial resection of the cyst wall was performed, showing serous xanthochromic fluid as the cyst contents. In the pathological specimen, short spindle cells and a palisade–like pattern were observed on hematoxylin and eosin staining (Figure 3B). This and strong immunostaining for S–100 protein indicated schwannoma. Hemosiderin deposition was also confirmed by Berlin–blue stain, suggesting microhemorrhage in the tumor (Figure 3C).
Figure 3: A) Intraoperative photography using a left transsylvian approach demonstrating the cystic mass with grayish capsule (T) continuously attached to the oculomotor nerve (cranial nerve III) in the carotid cistern. Histopathological examination of the surgical specimen shows: B) tumor comprising short spindle cells in a palisade-like pattern, corresponding to schwannoma (Hematoxylin and Eosin (HE), X40); C) hemosiderin deposition (blue signal), suggesting microhemorrhage in the tumor (Berlin-blue stain, X40). III; oculomotor nerve, IC; internal carotid artery, T; tumor.
After confirming successful evacuation of cyst fluid (Figure 4AC), the patient was discharged without improvement of oculomotor nerve palsy. Five weeks later, however, the patient was readmitted with sudden onset of right hemiparesis, disturbance of consciousness, and complete left oculomotor palsy. Emergent CT demonstrated intratumoral hemorrhage from the residual tumor with midbrain compression and associated hydrocephalus (Figure 4D). Following ventriculo–peritoneal shunt for hydrocephalus, a second operation was performed for subtotal removal of the tumor with sacrifice of the oculomotor nerve, since the nerve was behind the enlarged tumor and hematoma, and was damaged severely with total loss of function preoperatively. A small piece of residual capsule tightly adhering to the brainstem and internal carotid artery was left intact after the procedure. After improvement of clinical condition, the patient was transferred to a rehabilitation hospital, 12 months after initial onset. And even up to 58 months after the procedure, the patient experienced no further rebleeding although there was a loss of complete oculomotor function.
Figure 4: A-C) Postoperative unenhanced axial CT (A), T1-weighted MRI (B) and T2-weighted imaging (C) showing successful evacuation of cyst fluid. D) One month after first operation, axial unenhanced CT shows hematoma from the residual tumor compressing the brainstem and also causing non-communicating hydrocephalus.
Discussion
Schwannoma originating from the oculomotor nerve without neurofibromatosis is extremely rare. Such lesions are generally classified according to location, such as cisternal type (42%), cavernoussinus type (31%), cisterno–cavernous type (21%) and orbito–cavernous type (6%) [2,4]. The present case would be classified as cisternal type. The reported cases have mainly involved solid schwannoma, with the exception of two cases. One was mainly located in the lateral wall of the cavernous sinus and the tumor included a 10–mm round cyst [6]. The other was a relatively small mass, 15 mm in diameter and located in the cisternal portion, and exhibiting homogeneous hyperintensity on both T1– and T2–weighted imaging, as in the present case [5]. This latter case was preoperatively diagnosed as lipid–containing cystic craniopharyngioma, and subsequently identified pathologically as cystic schwannoma with microhemorrhage. These entirely cystic schwannomas, including the present case, may pose a diagnostic challenge.
A diagnostic clue in such cystic cases might be the presence of microhemorrhage, as observed in our case, since schwannoma is known to bleed intratumorally. In Vestibular Schwannoma (VS), progression of intratumoral microhemorrhage is reported to be associated with preoperative hearing loss [9]. In this study of 274 cases, most of the pathological specimen exhibited microhemorrhage, although major hemorrhage comprising >25% of the lesion was noted only in 23%. Several MRI studies have also suggested microhemorrhage in schwannoma [10–12]. More specific findings were reported by Thamburaj et al. who clearly demonstrated that 94% of VS showed signs of hemorrhage when examined on T2* imaging,suggesting this as a specific finding in differential diagnosis [13]. Such microhemorrhage, when repeated, has been considered to lead to cyst formation [14]. Indeed, cystic VS are reported to exhibit significantly more hemosiderin deposition than homogeneous solid VS [15]. In our case, repeated hemorrhaging, as verified by imaging and pathological findings (Figure 3C), was associated with cyst formation, which would also support this hypothesis.
Cyst itself, however, is not a specific finding in VS. According to the literature, the cyst formation comprises a mean of 5.7-48% of the VS, closer to 10% with more recent studies [16]. When associated with a large cyst, wall thickness and enhancement could offer diagnostic clues in favor of schwannoma when differentiating from other cysticlesions such as arachnoid cyst, neurenteric cyst, and epidermoid [16]. Based on these findings, our preoperative diagnosis was neurenteric cyst due to the homogeneous cyst and lack of wall enhancement.
Entirely cystic schwannomas with a lack of enhancement, particularly in the crural cistern, should be differentiated from other cystic lesions. Neurenteric cyst is one such lesion, although extremely rare [17]. This lesion is more commonly located within the spinal cord, but may develop intracranially; only 140 such intracranial cases have been reported. Although the posterior fossa is a representative location (70–90% of cases) for intracranial lesions, oculomotor nerve neurenteric cyst has been reported [7,18]. These tumors typically do not exhibit enhancement, but two reported oculomotor nerve neurenteric cysts displayed partial or complete rim enhancement [13,15]. While signal intensities are inconsistent on MRI, depending on the protein content, these tumors are usually slightly hyperintense relative to Cerebrospinal Fluid (CSF) on T1–weighted imaging. This difference in signal intensity from CSF allows distinction from arachnoid cyst. Neither hemorrhage nor nodules are present in neurenteric cyst, providing one clue for differentiation from oculomotor schwannoma.
Arachnoid cyst should also be considered, and usually exhibits isointensity with CSF on MRI. However, we found one report that documented hemorrhagic arachnoid cyst associated with third nerve palsy [19]. Although positive staining for Epithelial Membrane Antigen (EMA) was shown in that case, staining for S–100 was not described, leaving open the possibility of cystic oculomotor schwannoma. Since other cystic lesions should be differentiated from entirely cystic schwannoma in the oculomotor cistern or cavernous sinus, the imaging features are summarized in Table 1. Enhancement of the cyst wall [20], fluid intensity of the contents on MRI [21,22], presence of intracystic hemorrhage [23], and lesion location can all help in the differential diagnosis. In particular, we would like to emphasize the importance of intracystic hemorrhage as a clue in favor of schwannoma.
Neurenteric cyst
Arachnoid cyst
Cystic cranio-pharyngioma
Cystic pituitary adenoma
Rathke cleft cyst
Epidermoid cyst
Dermoid cyst
Cystic oculomotor schwannoma
Enhancement of the cyst wall [20]
usually none [17]
none
usually none
yes
none or thin cyst wall enhancement
usually none, minimal rim enhancement occurs in 25%
none
none
T1/T2-weighted signal intensity of the contents compared to
CSF [22]
is~slightly hyper/hyper
hypo/hyper (similar to CSF)
hyper~hypo on both T1/T2
vary as its contents [21]
hyper/ extremely hyper
hyper: hypo=1:1 / hyper: iso~hypo=7:3 vary as its contents [21]
iso~hyper on both T1/T2
hyper/ hypo~hyper (similar to fat)
hyper/ extremely hyper
Frequency
extremely rare
common
rare
rare
occasional
rare
extremely rare
extremely rare
Intracystic hemorrhage
none
usually none
rare
occasional
usually none [23]
none
none
yes
Calcification
usually none
none
yes
rare
none
yes, in 10-25%
yes
none
Remarks
FLAIR image show hyperintensity and restricted slightly on DWI
FLAIR image show hypointensity and not restricted on DWI
solid enhancing nodule presents usually in the cyst
enhancing rim presents in 50%
restricted on DWI located off the midline
located suprasellar at midline
located
along the oculomotor nerve
Metastatic tumor or parasitic lesion is excluded. FLAIR;fluid-attenuated inversion-recovery
Table 1: Differential diagnosis of whole entire cyst in oculomotor cistern or cavernous sinus.
Another issue to consider in a case like the present one is the surgical strategy. When the oculomotor function is intact preoperatively, primary goal of surgery would be preservation of nerve function because schwannoma is benign tumor. When sharp dissection seems difficult, subcapsular or subtotal resection is also recommended to preserve oculomotor function [8]. In previous surgical series of oculomotor schwannoma, oculomotor function became worse after surgery in 50% of case. However, recovery of its function is reported in 11% of case when conservative subtotal or partial resection was tried [2]. Given the age of the patient and partial impairment of the nerve function, we performed palliative partial cyst wall resection to relieve the mass effect to preserve the nerve function in the initial stage of surgery. However, the cyst rapidly expanded again due to repeated hemorrhage from the remaining cyst leading to total loss of the nerve function, which finally necessitated a second operation to resect the tumor with the oculomotor nerve. Gamma Knife Radiosurgery (GKR) after partial debulking might have been one treatment option, but there is only one oculomotor nerve schwannoma case subjected to GKR after surgery [24]. In our case, the tumor rebled as early as 5 weeks after surgery, so it remained unclear whether GKR could have prevented subsequent hemorrhage. Further investigation or experience of stereotactic radiosurgery for entirely cystic masses is needed. It is worth to notify that no other cases have been reported to harbor such oculomotor nerve schwannoma which rebled from the residual tumor necessitating a second operation. Based on our experience, we recommend at least subtotal removalof the cyst wall, preferably preserving the nerve function, to prevent rebleeding in entirely cystic schwannoma with signs of hemorrhage, even in elderly patients.
Conclusion
Entirely cystic oculomotor nerve schwannoma is a very rare entity. Intratumoral microhemorrhage is a possible cause of rapid growth within a short span, postoperative hemorrhage from the residual tumor, and cyst formation. In these cystic schwannomas, aggressive tumor resection should be considered as one f the surgical alternatives to avoid rebleeding from the residual tumor.
References
- Saetia K, Larbcharoensub N, Wetchagama N. Oculomotor nerve schwannoma: a case report and review of the literature. J Med Assoc Thai. 2011; 94: 1002-1007.
- Furtado SV, Hegde AS. Management of oculomotor nerve schwannomas in two different locations: surgical nuances and comprehensive review. Neurosurg Rev. 2012; 35: 27-34.
- Scheller C, Rachinger JC, Prell J, Alfieri A, Rampp S, Sel S, et al. Intraorbital oculomotor nerve schwannoma affecting only the parasympathetic fibers. Journal of neurological surgery Part A, Central European neurosurgery. 2013; 74: 120-123.
- Celli P, Ferrante L, Acqui M, Mastronardi L, Fortuna A, Palma L. Neurinoma of the third, fourth, and sixth cranial nerves: a survey and report of a new fourth nerve case. Surg Neurol. 1992; 38: 216-224.
- Katsumata Y, Maehara T, Noda M, Shirouzu I. Neurinoma of the oculomotor nerve: CT and MR features. J Comput Assist Tomogr. 1990; 14: 658-661.
- Kurokawa Y, Uede T, Honda O, Honmou O. Successful removal of intracavernous neurinoma originating from the oculomotor nerve--case report. Neurol Med Chir (Tokyo). 1992; 32: 225-228.
- Morgan MA, Enterline DS, Fukushima T, McLendon RE, Cummings TJ. Endodermal cyst of the oculomotor nerve. Neuroradiology. 2001; 43: 1063-1066.
- Ohata K, Takami T, Goto T, Ishibashi K. Schwannoma of the oculomotor nerve. Neurol India. 2006; 54: 437-439.
- Sughrue ME, Kaur R, Kane AJ, Rutkowski MJ, Yang I, Pitts LH, et al. Intratumoral hemorrhage and fibrosis in vestibular schwannoma: a possible mechanism for hearing loss. J Neurosurg. 2011; 114: 386-393.
- Curati WL, Graif M, Kingsley DP, King T, Scholtz CL, Steiner RE. MRI in acoustic neuroma: a review of 35 patients. Neuroradiology. 1986; 28: 208-214.
- Hashimoto H, Takemoto K, Inoue Y, Fukuda T, Shakudo M, Fukuda H, et al. [Magnetic resonance imaging of intracranial neurinomas]. Rinsho Hoshasen. 1988; 33: 21-25.
- Mikhael MA, Ciric IS, Wolff AP. MR diagnosis of acoustic neuromas. J Comput Assist Tomogr. 1987; 11: 232-235.
- Thamburaj K, Radhakrishnan VV, Thomas B, Nair S, Menon G. Intratumoral microhemorrhages on T2*-weighted gradient-echo imaging helps differentiate vestibular schwannoma from meningioma. AJNR Am J Neuroradiol. 2008; 29: 552-557.
- Park CK, Kim DC, Park SH, Kim JE, Paek SH, Kim DG, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006; 105: 576-580.
- Gomez-Brouchet A, Delisle MB, Cognard C, Bonafe A, Charlet JP, Deguine O, et al. Vestibular schwannomas: correlations between magnetic resonance imaging and histopathologic appearance. Otol Neurotol. 2001; 22: 79-86.
- Piccirillo E, Wiet MR, Flanagan S, Dispenza F, Giannuzzi A, Mancini F, et al. Cystic vestibular schwannoma: classification, management, and facial nerve outcomes. Otol Neurotol. 2009; 30: 826-834.
- Gauden AJ, Khurana VG, Tsui AE, Kaye AH. Intracranial neuroenteric cysts: a concise review including an illustrative patient. J Clin Neurosci. 2012; 19: 352-359.
- Okunaga T, Tsutsumi K, Hayashi T, Nagata I. Endodermal cyst of the oculomotor nerve: case report. Neurosurgery. 2006; 58: E994.
- Ide C, De Coene B, Gilliard C, Pollo C, Hoebeke M, Godfraind C, et al. Hemorrhagic arachnoid cyst with third nerve paresis: CT and MR findings. AJNR Am J Neuroradiol. 1997; 18: 1407-1410.
- Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006; 239: 650-664.
- Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A. MR differentiation of adamantinous and squamous-papillary craniopharyngiomas. AJNR Am J Neuroradiol. 1997; 18: 77-87.
- Choi SH, Kwon BJ, Na DG, Kim JH, Han MH, Chang KH. Pituitary adenoma, craniopharyngioma, and Rathke cleft cyst involving both intrasellar and suprasellar regions: differentiation using MRI. Clin Radiol. 2007; 62: 453-462.
- Binning MJ, Liu JK, Gannon J, Osborn AG, Couldwell WT. Hemorrhagic and nonhemorrhagic Rathke cleft cysts mimicking pituitary apoplexy. J Neurosurg. 2008; 108: 3-8.
- Kim IY, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Gamma Knife surgery for schwannomas originating from cranial nerves III, IV, and VI. J Neurosurg. 2008; 109 Suppl: 149-153.