Research Article
Austin Neurosurg Open Access. 2014;1(4): 1020.
Meningioma Removal Using Sodium Fluorescein as a Fluorescent Marker
da Silva CE1*, da Silva VD2 and da Silva JLB3
1Department of Neurosurgery and Skull Base Surgery, Hospital Ernesto Dornelles, Brazil
2Department of Pathology and Radiation, Pontifical Catholic University of Rio Grande do Sul- PUCRS, Brazil
3Service of Hand Surgery and Reconstructive Microsurgery, Pontifical Catholic University of Rio Grande do Sul- PUCRS, Brazil
*Corresponding author: Carlos Eduardo da Silva, Department of Neurosurgery and Skull Base Surgery, Hospital Ernesto Dornelles, A. Independência 1801/box 26, Porto Alegre, 90160-093, Brazil
Received: June 28, 2014; Accepted: August 22, 2014; Published: August 26, 2014
Abstract
Meningiomas are a neurosurgical challenge and are related to the historical development of neurosurgery. Efforts to achieve radical removal to promote better disease control remain the goal. The authors present an innovative experience with sodium fluorescein, a fluorescent marker in meningioma surgery and perform critical analysis of this introductory study.
A retrospective study that includes twenty-two meningiomas operated on using sodium fluorescein as a fluorescent marker between2009 and 2014 was carried out.After the initial dissection, a dose of 1g of the sodium fluorescent 20% was injected into a peripheral vein and an enhancement of SF by meningiomas was used as a fluorescent guide for tumor resection. The fluorescent effect of the dye was photographed and submitted to digital analysis.
The group of twenty-two meningiomas was composed as follows: seventeen skull base lesions and five convexity meningiomas. The enhancement in all tumors was strongly positive. In skull base meningiomas contrast between tumors and cranial nerves was observed. Dural tail was enhanced in an irregular fashion both in convexity and skull base meningiomas. Simpson grade zero was achieved in one convexity meningioma; Simpson grade 1 was obtained in 8 skull base meningiomas and in 3 convexity tumors; Simpson grade 2 was obtained in 8 skull base tumors and one convexity lesion; Simpson grade 3 was achieved in one skull base meningioma. Sodium fluorescein is a useful and universally available fluorescent marker for meningioma surgery.
Keywords: Fluorescent marker; Fluorescent guided surgery; Meningioma; Simpson grade
Abbreviations
SF: Sodium Fluorescein; MRI: Magnetic Resonance Imaging; BBB: Blood-Brain Barrier
Introduction
Meningiomas are a neurosurgical challenge related to the historical development of neurosurgery. In skull base meningiomas, these challenges are enormous due to the neurovascular structures involved in the treatment of these lesions. In convexity tumors, efforts to achieve an extended removal of the dural tail are a major concern. In meningiomas, surgical treatment is one of the most valuable options. Radical removal, to promote better disease control, continues to be a goal.
Sodium fluorescein was first used as a fluorescent marker in neurosurgery in 1948, dealing with different types of tumors [1]. Subsequently, the use of SF and others fluorescent markers, particularly those that address surgical treatment of glioblastoma multiforme and metastatic disease in the brain, has been described in literature [2-3]. In 2010, the use of SF was described for the first time as an adjuvant for the surgical resection in skull base lesions [4].
The authors present an innovative experience with sodium fluorescein as a fluorescent marker in meningioma surgery and show a critical analysis, pointing out both the limits and advantages of such a method.
Materials and Methods
A retrospective study was designed and carried out. This study included twenty-two patients with skull base and convexity meningiomas who underwent operations between December 2009 and March 2014. The criteria for inclusion in the study were the use of SF as a fluorescent marker during the surgery and histopathological diagnosis of the meningioma. All patients were informed of the trans-operative use of sodium fluorescein to enable viewing of the tumors during the surgical procedure. Having been informed, the patients provided written consent prior to the procedure.
Initial dissections were performed and, after exposuring of the tumors, an initial digital photo was taken through the optical lens of a microscope. The digital camera used in skull base meningiomas was a Sony TM model DSC-W90 with 8.1 megapixels; macro activation was on, and the internal flash was off. In convexity meningiomas a digital camera Medi Live Carl Zeiss TM 1 CCD, with manual white balance was used for the photos. The light-source for the pictures was the same as that used for the microscope, and the captured images were seen by the surgeon, without the use of any special filters.
A dose of 1 g of 20% SF was injected into a peripheral vein and pictures were obtained 10 minutes after SF injection, using the same technique as described above.
Results
The group of twenty-two meningiomas was composed of the following types of tumors: one cavernous sinus, one tuberculum sellae, one cranio-cervical junction, 2 olfactory groove, 2 tentorial, 2 anterior clinoid, 2 temporal floor,3 petroclival,3 sphenoid wing, and 5 frontal. Table 1 illustrates the series and characteristics.
SITE
SEX
AGE (years)
NEUROLOGIC DEFICIT
SIMPSON GRADE
Frontal
Female
37
None
zero
Frontal
Male
62
None
1
Frontal
Female
60
None
1
Frontal
Female
57
None
1
Frontal
Male
72
None
2
CS
Male
60
None
2
CCJ
Female
72
None
2
TF 1
Female
28
None
2
TF 2
Female
48
Abducens ( T )
3
TS
Female
28
None
1
A CLIN 1
Female
42
None
1
A CLIN 2
Female
60
None
1
TENT 1
Female
62
None
1
TENT 2
Female
73
None
2
OG 1
Female
69
Olfactory ( D )
2
OG 2
Female
72
Olfactory ( D )
1
SW 1
Female
62
None
1
SW 2
Female
60
None
1
SW 3
Female
41
Oculomotor (T)
1
PC 1
Female
56
Hemiparesis (D)
2
PC 2
Female
48
None
2
PC 3
Female
61
None
2
Table 1: Summary of 22 Cases of Dural-Based Cavernous Angiomas outside the Middle Fossa since 1992.
Discussion
Moore et al. first investigated the use of sodium fluorescein in neurosurgery in 1948 [1]. Kuroiwa et al. has tested the applicability of SF to the surgical removal of glioblastoma multiforme and Okuda et al. has used SF in metastatic brain lesions [2-3].
In meningioma surgery, radical removal is extremely important, thereby achieving better disease control. Apart from a study, which fails in correlate the Simpson grade of recurrence of meningiomas [5,6], abundant evidence in literature points to the correspondence of the radical surgical resection and the control or cure of the tumor [7-13]. In convexity meningiomas, such efforts include aggressive identification and removal of the mass and dural attachment. The application of sodium fluorescein in convexity meningiomas enabling the identification of a dural tail was recently described and gave interesting results in terms of dural enhancement around the mass, suggesting a possible role of the dye in future applications as an adjuvant for radical removal of such tumors [14].
The use of SF during cranial base meningioma surgery was an extension of a previous study that investigated SF for skull base tumors [4]. In that study, the clinical effect of enhancing the skull base tumors with SF was found to be strongly positive and half of the six tumors included were meningiomas. More recently, a series with twelve meningiomas of the cranial base enhanced by SF were shown, with very consistent results in digital analysis of the SF enhancement of the tumors [15].
In all cases of the present series, the dose of 1 g of SF 20% was the same as the former studies [4,13,15]. The effect of tumor enhancement can be clearly visualized 10mins after administration of IV SF and this persists for several hours. The dye was observed under standard white light microscopes, and such aspect can be reproduced in any department (Figures 1 & 2). However, it is possible that the development of special filters, helping the SF wavelength identification could improve the contrast between the meningiomas and the surrounding structures enhanced by the fluorescein. In fact, a recent study tested the SF guided removal of malignant tumors under a YELLOW 560 nm surgical microscope filter and gave interesting results using lower doses of SF [16].
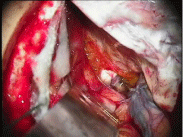
Figure 1: Tuberculum sellae meningioma-note the SF enhancement of the tumor and the contrast between the mass and the right optic nerve.
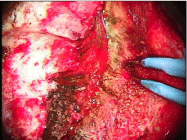
Figure 2: Left sphenoid wing meningioma - observe the SF enhancement of the lesion pointed by the bipolar forceps.
The pattern of meningioma enhancement varies from case to case. In a general sense, the skull base lesions seem to have a stronger effect inside the mass, while the convexity lesions present a more visible dural impregnation around the tumor. It is possible that differences of illumination, especially of those between skull based and convexity lesions, play a role in the visual perception of the enhancement using SF. The angles of the microscopes` light are variable and the exposure of the lesion, dura and surrounding tissues are also distinct. Such aspects should be involved in the SF visualization of the surgeon.
The vascular characteristics of the meningiomas are probably a determinant for tumor enhancement. Lesions with more intense gadolinium capture on MRI tend to be more affected by SF enhancement. Probably BBB disruption is involved in the enhancement of the meningiomas. In convexity meningiomas, the dural tail presents a “spot fashion enhancement”, visible at higher magnifications. In all five cases included in the present series, the histopathology confirms the tumors` involvement in the dural region. Another interesting observation was that the dural enhancement was seen to be more intense around the venous sinuses invaded by meningiomas. In two cases, one of an atypical meningioma of the convexity with superior sagittal sinus involvement, and another a tentorial meningioma with occlusion of the transverse sinus, enhancement around the sinuses were clearly observed.
In skull base lesions, especially in petroclival and cavernous sinus meningiomas, the SF enhancement helped to create a contrast between the tumors and the cranial nerves, this was very interesting for the anatomical preservation of the neural structures during tumor resection. An initial study of such a technique was recently described [17]. Cranial nerve identification and functional preservation is proving to be a promising field for research expansion of the SF and other fluorescent markers in skull base meningiomas.
All fluorescent markers present limitations and SF was used with critical concern. The removal of the convexity meningiomas and their dural tail were guided by MRI image analysis. The possibility of a false negative should be kept in mind when applying fluorescent markers in neoplasm surgery. In skull base tumors, trans-operative neurophysiology and neuronavigation are crucial for neural preservation and SF should be used as a complimentary tool.
Conclusion
The initial experience of the SF application in meningioma surgery was very positive. SF is a safe dye, universally available at a low cost. Further studies should test special filters, compare SF and other fluorescent markers, observe the neurovascular preservation and Simpson grade results, in order to validate and include SF as a useful tool in meningioma surgery.
References
- Moore GE, Peyton WT. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg. 1948; 5: 392-398.
- Kuroiwa T, Kajimoto Y, Ohta T. Comparison between operative findings on malignant glioma by a fluorescein surgical microscopy and histological findings. Neurol Res. 1999; 21: 130-134.
- Okuda T, Kataoka K, Taneda M. Metastatic brain tumor surgery using fluorescein sodium: technical note. Minim Invasive Neurosurg. 2007; 50: 382-384.
- da Silva CE, da Silva JL, da Silva VD. Use of sodium fluorescein in skull base tumors. Surg Neurol Int. 2010; 1: 70.
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957; 20: 22-39.
- Sughrue ME, Kane AJ, Shangari G, Rutkowski MJ, McDermott MW, Berger MS, et al. The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg. 2010; 113: 1029-1035.
- Cusimano MD, Sekhar LN, Sen CN, Pomonis S, Wright DC, Biglan AW, et al. The results of surgery for benign tumors of the cavernous sinus. Neurosurgery. 1995; 37: 1-9.
- DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994; 81: 245-251.
- Erkmen K, Pravdenkova S, Al-Mefty O. Surgical management of petroclival meningiomas: factors determining the choice of approach. Neurosurg Focus. 2005; 19: E7.
- Knosp E, Perneczky A, Koos WT, Fries G, Matula C. Meningiomas of the space of the cavernous sinus. Neurosurgery. 1996; 38: 434-442.
- O'Sullivan MG, van Loveren HR, Tew JM Jr. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997; 40: 238-244.
- da Silva CE. Surgical treatment of olfactory groove meningiomas. J BrasNeurocir 2006; 17: 25-30.
- Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. 2012; 117: 121-128.
- da Silva CE, da Silva VD, da Silva JL. Convexity meningiomas enhanced by sodium fluorescein. Surg Neurol Int. 2014; 5: 3.
- da Silva CE, da Silva VD, da Silva JL. Sodium fluorescein in skull base meningiomas: a technical note. Clin Neurol Neurosurg. 2014; 120: 32-35.
- Schebesch KM, Proescholdt M, Höhne J, Hohenberger C, Hansen E, Riemenschneider MJ, et al. Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery-a feasibility study. Acta Neurochir (Wien). 2013; 155: 693-699.
- da Silva CE, da Silva VD, da Silva JLB. Skull base meningiomas and cranial nerves contrast using sodium fluorescein: A new application of an old tool. J NeurolSurg B 2014.