
Review Article
Austin Neurosurg Open Access. 2024; 10(1): 1075.
Anatomical Variability of the Inferior Fronto-Occipital Fasciculus: A Systematical Review
Giuseppina Bevacqua¹*; Carlo Conti¹; Eleonora Becattini¹; Alessandro Ciampini¹; Marco Ciavarro²
1Department of Neuroscience, Neurosurgery Unit, Azienda Ospedaliera Santa Maia di Terni, Viale Tristano di Joannuccio, Italy
2Department of Neuroscience, Neurosurgery Unit, I.R.C.C.S. Neuromed, Italy
*Corresponding author: Giuseppina Bevacqua Department of Neuroscience, Neurosurgery Unit, Azienda Ospedaliera Santa Maia di Terni, Viale Tristano di Joannuccio, 05100 Terni (TR), Italy. Email: giusy.bevacqua3@gmail.com
Received: April 01, 2024 Accepted: May 01, 2024Published: May 08, 2024
Abstract
The Inferior Frontal Occipital Fasciculus (IFOF) is a lengthy associative white matter pathway, linking the posterior cortex to the frontal areas by traversing through the external/extreme capsule area. This review aims to analyze the structural characteristics of the IFOF and its cortical terminations. The review was guided by PRISMA protocol and from 469 articles screened 53 were retained for full-text examination, of which 10 finally fulfilled our criteria to be included. Whereas there is a broad consensus regarding fibers reaching the pars orbitalis and pars triangularis of the inferior frontal gyrus and orbital frontal cortex, there is discrepancy regarding the termination in other frontal regions, specifically the pars opercularis or the middle frontal gyrus. Similarly, defining cortical connections in the posterior terminations of the IFOF poses challenges since the terminations were found only with the lingual gyrus, whereas the other cortical connections similarly remain less consistent. In particular, the percentage of termination in cuneus, middle and inferior occipital gyrus and fusiform area lacking consistent agreement among examined studies. Moreover, whereas first studies overlooked parietal connections, particularly with the superior parietal lobule, more recent evidence has strengthened the relevance. Finally, projections to areas like the superior occipital gyrus, calcarine cortex, and occipital pole show inconsistency and variability in identified terminations. In the last years anatomical dissection studies and diffusion-weighted tractographic methods have increased knowledge about structure-function of IFOF, however, the high variability in identified its subcomponent and cortical termination poses a challenge on data integration and interpretation.
Introduction
The Inferior Fronto-Occipital Fasciculus (IFOF) stands as one of the longest association fiber tracts of the ventral stream, establishing connections between the frontal lobe and diverse regions of the posterior cortex [1]. Despite initial records of the IFOF dating back to 1822 [2]; research on this issue was limited until the early 2000s [3-5]. Recent resurgence in interest surrounding this ventral fasciculus can be attributed chiefly to the revival of postmortem anatomical dissections [6,7] and advancements in diffusion tensor imaging, enabling indirect visual representation of fiber tracts [8-13]. A thorough examination of the available literature unveils discrepancies in the anatomical depictions of IFOF, both in Diffusion Tensor Imaging (DTI) and white matter dissection studies, resulting in significant gaps concerning its subcomponents and its structure. Notably, descriptions of the exact course and cortical connections of the IFOF vary significantly and diverse models detailing the IFOF connections exist in current literature. This review aims data on the topological organization of the IFOF, including historical findings on fiber dissection and more recent DTI and dissection studies that suggest a complex, multi-layered structure.
Literature Review
This systematic review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (PRISMA) [14]. An extensive search of English-language literature was conducted using PubMed, Scholar, and Scopus, yielding a total of 469 articles. The search terms were: (“inferior front occipital fasciculus” OR “IFOF”) AND (“Anatomy” OR “dissection”) AND (“DTI” OR “tractography” OR “Diffusion Tensor Imaging”)). A rigorous screening process was initiated to eliminate duplicate records and filter the articles based on their titles, abstracts, and subsequent full texts, ensuring the selection of studies pertinent to the subject matter. Emphasis was placed on anatomical and laboratory studies focusing on the assessment of IFOF fiber orientation, cortical origin, and terminations using fiber dissection and/or DTI (Diffusion Tensor Imaging). Manuscripts focused on intraoperative mapping for tumor lesions and studies that did not specifically report the frontal and/or parieto-temporo-occipital cortical areas involved in IFOF were excluded. To evaluate cortical connections in alignment with the guidelines described by Destrieux et al. (2017) according to the Terminologia Anatomica, a meticulous assessment of the abstracts was conducted, followed by the application of predefined inclusion and exclusion criteria. To visualize the article selection process, a PRISMA flow diagram (Figure 1) was constructed, illustrating the number of articles at each stage of data acquisition, the excluded articles, and the specific reasons for their exclusion. A total of 275 records were retrieved. The titles and abstracts of 53 records were screened. The records were filtered by study type, pathological study, and missing result. During exclusion criteria application and full-text screening, 32 records were excluded, with 21 remaining articles from 1997 to June 2023, including anatomical dissection and DTI studies. To review the available data about the IFOF, we started describing the anatomy and then we highlight its cortical areas connections in the frontal and posterior(temporo-parieto-occipital) cortex. Attention was also paid to hypotheses on anatomical structure and possible functional implications.
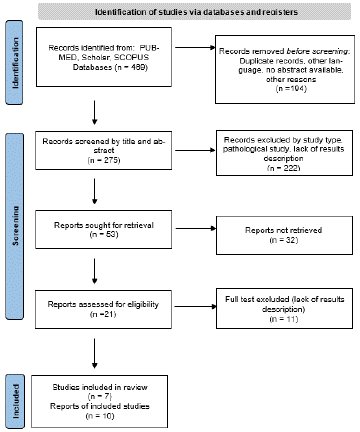
Figure 1: A PRISMA flow diagram showing the flow of information through the different phases of the systematic review.
Results
Historical Descriptions
The first citation of a direct connection between the frontal and occipital lobes is contained in Burdach's 1822 [2] description of the Inferior Longitudinal Fasciculus (ILF), a bundle connecting the occipital and temporal lobes, first described by Reil in 1809 as a projection pathway [15]. Burdach not only recognized the corticocortical (or associative) nature of the ILF, but also described a subcomponent of the tract associated with the frontal lobe without coning the term “iFOF” [2]. Although Adrien Charpy's (1895) [16] description of the fronto-occipital connection was comparable to that of Karl Burdach (1822), Jules Dejerine was the first to separate the fronto-occipital tract from the ILF and consider it as a separate bundle [3]. Curran was the first to use the term IFOF to distinguish it from its dorsal counterpart the superior fronto-occipital fasciculus (sFOF). He defined the IFOF as “a large associating bundle of fibres uniting, as its name indicates, the occipital with the frontal lobe. It also contains fibres, which join the frontal lobe with the posterior part of the temporal and parietal lobes. […] From all parts of the frontal lobe the fibres of this fasciculus can be traced converging to a single bundle which swings round the lower external side of the nucleus lentiformis, at which place it appears as a distinct bundle […]”. (1909, p. 652) [5]. Recent advances in Diffusion Tensor Imaging (DTI) technique, allowing ‘‘in vivo’’ dissection of the human brain white matter [8], and more recent anatomical and stimulation studies demonstrated its existence and its posterior origin from dorsal parieto-occipital and basal temporo-occipital areas. Nonetheless, the exact anatomo-functional organization of the terminations of the IFOF remains controversial [17].
Controversial Topics
The existence and location of the IFOF were the subject of contentious debate in the historical literature [1]. The IFOF debate was influenced by anatomical studies of animals. Schmahmann et al., questioned the existence of IFOF in rhesus monkeys because it was not detected by tracing methods. They suggested that the IFOF observed in human tractography may be due to a diffusion-weighted artifact explained by the proximity of the ILF to the UF and extreme capsule [1,11]. However, further studies have documented the existence of this tract. Mars et al. [18], showed a persuasive anatomic similarity between the macaque and human connections, suggesting the presence of a common ventral pathway directly connecting the frontal and occipital lobes. Sarubbo et al. [19] recently conducted a study using post-mortem diffusion MRI tractography and Klingler micro-dissection to provide evidence for the existence of bilateral fiber tracts in non-human primate corresponding to human IFOF in trajectory, topological organization and cortical end fields [19].
Furthermore, indirect evidence in support of the existence of IFOF in humans can be found in studies using other methods. Rudrauf et al. [20] investigated the activity within the ventral visual processing in humans during the visualization of emotional scenes using Magnetoencephalography (MEG). In this way they found a latency of 100 msec between early response in visual areas (V2eV3eV4) and the orbitofrontal and ventro-medial prefrontal cortex, suggesting that this signal is a results of a long-range monosynaptic association fiber (inferior fronto-occipital connections). These results are consistent with previous electrophysiological studies on visual perception [21,22]
Finally, IFOF has been consistently identified in postmortem sections of normal human brains, and its presence has been reported in over 50 least dissected hemispheres [6,7,23].
Anatomical Description Derived from Postmortem Fibers Dissections
All studies based on ex-vivo microdissection agree have detected a peculiar waypoint, known as the 'stem' of IFOF, a region of white matter where all the fibers of a fascicle are collected. The IFOF stem is located in the white matter of the ventral third of the external capsule, just medial to the putamen and ventral to the claustrum [6,7,24]. During the first step of dissection, removing of specific parts of the insula, including the apex, long gyri, central sulcus, and short gyri, the complex structure of the short fibers in the insulo-opercular and claustro-opercular areas within the extreme capsule, external capsule, and dorsal claustrum (postero-superior section) becomes visible. This region can be divided into two distinct sections. The posterior part shows an antero-inferior orientation and encompasses the insulo and claustro-opercular fibers, the dorsal claustrum, and the dorsal part of the external capsule. On the other hand, the anterior segment displays a postero-inferior orientation and includes the ventral external capsule housing the VC, UF, and IFOF, moving from the outer side towards the inner side [6,7,17,24]. The lower part of the external capsule holds the UF (positioned superficially and ventrally) and the IFOF (located deeper and dorsally). Particularly, the IFOF, forming the rear two-thirds of the ventral external capsule, widens as it moves towards the frontal lobe, taking on a fan-shaped 60° radiation pattern. At the level of the anterior temporal lobe, a clear differentiation between the IFOF and the fibers of the uncinate fasciculus was noticeable. The IFOF tracts extend towards the posterior temporal and occipital lobes, while the fibers of the uncinate fasciculus curve towards the anterior temporal lobe.
Within the temporal area, the IFOF courses along the top of the temporal horn, positioned superiorly and laterally in relation to the optic radiations. Additionally, discernible distinctions were observed between the IFOF fibers (oriented anterior-posteriorly) and those of the arcuate fasciculus (superficial and oriented superior-inferiorly) in the posterior temporal region. Another section of the IFOF, characterized by its lower and posterior orientation, travels above the temporal horn, progressing posteriorly along the lateral surface of the atrium and occipital horn before reaching the occipital and basal temporal lobes [6,24].
Cortical Distributions of the IFOF Anterior Connections
From the temporal stem, the fibers run to the frontal lobe creating a 60° fan-shaped radiation. According to all studies analyzed reported the fibers reach the pars orbitalis, and triangularis of the IFG (100%) and OFC (94%). The anterior cortical distribution includes also MFG (72%) and SFG (61.2%). There is disagreement regarding the connection to the pars opercularis of the IFG, which was not reported in early dissection and DTI studies [6,7]. Regarding the connections with the frontal pole, there is limited evidence and discrepancies. Most studies do not take this area into consideration. Only two studies provide a detailed description of the distribution percentages of the frontal pole for both hemispheres [13,26]. Despite subsequent studies, there is still no evidence of localization at the frontal pole [27,28] (Table 1).
Pars Orbitalis
Pars Triangularis
OFC
SGF
MFG
Pars opercularis
Frontal pole
NA
TOT Hemisheres
Martino et al.
14
14
Sarubbo et al.
11
11
11
11
11
11
Forkel et al.
66
66
66
66
66
66
Caverzasi et al.
40
40
35
39
40
28
36
40
Wu et al.
20
11
20
18
12
11
20
20
Hau et al.
120
120
106
33
69
120
Panesar et al.
60
60
60
60
60
60
60
Tot
317
308
298
227
192
165
56
14
331
%
100
97
94
73
60
52
18
Table 1: Cortical distributions of the IFOF anterior connections.
Cortical Distributions of IFOF Posterior Connections
The main posterior connections have been identified in the lingual gyrus (85%). The remaining cortical distribution are not well defined. The IFOF is also connected to middle occipital gyrus (62%) and inferior occipital gyrus (61%), cuneus (58%) and fusiform gyrus at 43%. Although the initial studies did not find any connections, it has been extensively proven that there are indeed connections between IFOF and SPL. This clarification can help us understand the role of IFOF in the processes of reading and writing [29]. Superior occipital gyrus (33,3%) and calcarine cortex (30%) and Occipital pole (26%) are marginal projections. Connections with angular gyrus (22,7%) were described and can explain language role of IFOF. Only one study reported projection to superior temporal gyrus (22%) [28]), especially in the left hemisphere. There reported less 5% of precuneus (4,9%), inferior temporal gyrus (2,8%), and post central gyrus (1.5%). The connection with the inferior temporal gyrus was described in the first study in over 50 % of cases. However, all subsequent studies have not confirmed this finding. Only Wu et al. [26] described one case, and the same authors found a connection in 5 out of 20 cases with post central gyrus. The cortical distributions of the IFOF at the Middle Temporal Gyrus (MTG) has been delineated in a limited number of studies. Notably, this termination has been highlighted in only two specific investigations. Wu et al. observed a connection to the Middle Temporal Gyrus (MTG) in a one case, specifically involving the right hemisphere [26]. Subsequently, Hau and colleagues [28] reported occurrences of this connection to the MTG, with findings revealing left hemisphere involvement in 33% of subjects and right hemisphere involvement in 52% of cases. This particular association holds potential relevance in neuro-oncology, as it aligns with evidence suggesting that stimulation of the middle-posterior component of the right IFOF may induce left spatial neglect [30] (Table 2).
LG
MOG
IOG
Cu
SPL
FG
SOG
Ca
OP
AG
STG
Pcu
ITG
Post CG
Other
NA
TOT Hemisheres
Martino et al.
14
8
8
14
Sarubbo et al.
1
1
1
10
11
Forkel et al.
66
66
66
66
66
66
Caverzasi et al.
40
40
19
40
22
40
40
37
40
Wu et al.
19
19
19
19
11
12
14
19
10
1
1
5
1
20
Hau et al.
95
96
64
32
66
26
26
71
51
120
Panesar et al.
53
44
32
50
46
5
53
58
15
60
Tot
274
199
196
186
163
140
107
98
85
73
16
9
5
52
10
331
%
85
62
61
58
51
44
33
31
26
23
5
3
2
16
Table 2: Cortical distributions of the IFOF posterior connections.
Does the IFOF Have an Asymmetric, Multilayer Structure?
Recent studies based on Klingler's dissection method and fiber tractography not only confirmed the classical descriptions of the direct connection between the occipital and frontal regions but also proposed the existence of subcomponents of this association tract. The first study was conducted by Martino [6], identified two distinct components of the IFOF: (i) a superficial and dorsal subcomponent connecting the frontal lobe with the superior parietal lobe and the posterior portion of the superior and middle occipital gyri, and (ii) a deep and ventral subcomponent connecting the frontal lobe with the posterior portion of the inferior occipital gyrus and with the posterior basal temporal region. As these two areas (occipital associative extrastriate cortex and temporo-basal region) are known to be involved in semantic processing, the present findings are consistent with the hypothesis of a functional role of the IFOF in the semantic system, previously supported by intrasurgical electrostimulation studies [25,7], confirming the organization of the IFOF fibers into two main components, superficial and deep, and combining the dissection results with the DTI study, proposed that the frontal endings of the superficial layer, including the pars triangularis and orbitalis of Inferior Frontal Gyrus, (IFG) are distinct from the frontal endings of the deep layer, that it is divided into three bundles directed to the orbital, middle and dorsal frontal gyri. However, in contrast to Martino et al. they did not observe any part of the IFOF within the parietal lobe. Caverzasi et al. [13] using fiber-tracking dissection of both left and right IFOF with Q-ball residual bootstrap reconstruction of High-Angular Resolution Diffusion Imaging (HARDI) methods in 20 healthy subjects, confirmed the subdivision into two components described by Martino et al. In all subjects, they found occipital terminations in both hemispheres, particularly towards the pericalcarine and lateral occipital cortex. However, in contrast to Martino et al.'s study, terminations were also found towards the lingual gyrus, which was previously reported to only be reached by optic radiation terminations. In addition, they also found terminations towards the cuneus (30% of patients in the left hemisphere and 60% in the right hemisphere) and in line with the findings of Martino et al, they found extra occipital terminations posteriorly towards the temporal and parietal lobes. IFOFq showed temporal terminations only in the posterior part of the fusiform gyrus (bilateral in 55% of subjects). This area has been implicated in verbal and facial recognition by contrast no connections to the inferior temporal gyrus was found, whereas all subjects showed bilateral IFOFq terminations towards the superior parietal and angular gyrus. By contrast, through DSI-based tractographic, based on to frontal anatomic distributions, Wu et al. [26], have identified five potential subcomponents of the IFOF connecting the different cortical and subcortical regions. IFOF-I originated from the frontal polar cortex, FOF-II originated from the orbito-frontal cortex, IFOF-III originated from the inferior frontal gyrus, IFOF-IV originated from the middle frontal gyrus and finally IFOF-V originated from the superior frontal gyrus. This research aimed to categorize the IFOF based on its ending patterns, yet their framework did not incorporate the previously described organization into superficial and deep layers. Furthermore, subsequent studies [27] have suggested that the identification of an 'IFOF-V' was an observation of claustro-cortical fibers located within the dorsal external capsule, due to their markedly oblique angle. Finally, Panesar et al. [27], proposed a trifascicular gross IFOF structure consisting of a superficial ventrolateral layer originating from BA 44, 45, 47; a deep dorsomedial subfascicle originating from BA 8, 9, 10; and a deep ventromedial subfascicle originating from BA 11.
Discussion
The Inferior Frontal Occipital Fasciculus (IFOF) has been the subject of significant research endeavors aimed at unraveling its anatomical structure and potential functions within the brain. This review consolidates recent studies and historical perspectives on the IFOF, aiming to synthesize its anatomical variability and hypothesized involvement in neurological and neurodevelopmental disorders.
The IFOF, an extensive white matter tract, serves as a crucial pathway connecting various brain regions, including the frontal lobe with the temporal-occipital (and parietal) areas, facilitating complex cognitive functions. However, the historical records of the IFOF, dating back to the 1800s, experienced intermittent attention until the emergence of advanced tractographic imaging techniques and postmortem anatomical dissections in the early 2000s.
A major highlight of the review is the disparities observed across studies regarding the anatomical depiction of the IFOF. These inconsistencies encompass variations in diffusion tensor imaging (DTI) representations and white matter dissection studies, contributing to significant gaps in understanding the subcomponents and functionalities of this intricate pathway.
Historical descriptions from Burdach [3], Dejerine [4], and Curran [5] among others laid the groundwork for identifying and distinguishing the IFOF from other fiber tracts. However, controversy persisted concerning its existence and location, influenced by anatomical studies of animals, which questioned its presence in rhesus monkeys.
Despite the initial skepticism, recent evidence from studies like Mars et al. [18] and Sarubbo et al. [19] supported the existence of a ventral pathway directly connecting the frontal and occipital lobes in both human and monkey brains. These findings were corroborated by postmortem sections of human brains and studies utilizing electrophysiology and functional imaging. An important aspect of the review involves the discussion on the potential multilayered structure of the IFOF. Studies by Martino et al. [6], Sarubbo et al. [7], Caverzasi et al. [13], Wu et al. [26], Panesar et al. [27], among others, indicated the subdivision of the IFOF into distinct components or layers based on anatomical dissections, tractography, and cortical connections. These subdivisions ranged from superficial and dorsal layers to deep and ventral layers, exhibiting diverse terminations in various brain regions, including the parietal, occipital, and frontal lobes.
Specifically, Martino's pioneering work identified two primary components of the IFOF, delineating a superficial and dorsal subcomponent linking the frontal lobe to specific areas of the parietal and occipital gyri, alongside a deep and ventral subcomponent connecting the frontal lobe to regions like the inferior occipital gyrus and the basal temporal region. These findings aligned with previous hypotheses associating the IFOF with semantic processing, supported by intrasurgical electrostimulation studies [30-33].
Moreover, subsequent investigations by Sarubbo et al. [7] and Caverzasi et al. [13], confirmed and expanded upon Martino's subdivisions, proposing distinctions between the frontal endings of the superficial and deep layers of the IFOF, and reinforcing the organization into two components. However, discrepancies emerged regarding the presence of the IFOF within the parietal lobe, with Sarubbo et al. not observing such connectivity, deviating from Martino's earlier observations.
Furthermore, Wu et al. [26] and Panesar et al. [27] introduced alternative conceptualizations of the IFOF's subcomponents, emphasizing its connectivity across various cortical regions, delineating subfascicles originating from different Brodmann areas. While these models differed from previous descriptions, they offered additional perspectives on the complex organization of the IFOF. Overall, the diversity of findings underscores the multifaceted nature of the IFOF, exhibiting variable terminations in cortical and subcortical regions. The proposed subcomponents and subfascicles shed light on its intricate connectivity patterns. The presented findings regarding the cortical distributions of the IFOF's anterior and posterior connections highlight several critical issues and discrepancies within the research (Figure 2). Regarding the anterior connections of the IFOF, the identified cortical distributions exhibit inconsistencies among various studies. There is substantial agreement concerning the fibers reaching the pars orbitalis and triangularis of the IFG and OFC. However, discrepancies arise concerning the involvement of other areas such as the pars opercularis of the IFG, which was not consistently reported in earlier dissection and DTI studies. Additionally, frontal pole involvement was only observed in a limited number of studies utilizing tractography methods, indicating a lack of consensus across different research methodologies. Similarly, the posterior terminations of the IFOF present challenges in defining cortical connections. While there is a significant association with the lingual gyrus, the delineation of other cortical areas remains less clear. The bundle's connections to the cuneus, middle occipital gyrus, inferior occipital gyrus, and fusiform gyrus vary in reported percentages, lacking consistent agreement among studies. The association established between these regions, particularly the fusiform gyrus, substantiates the theory regarding the involvement of the IFOF in facial recognition [34]. Notably, several studies propose the participation of the IFOF in the identification of emotional facial expressions [35]. This proposition gains support from observations in individuals with congenital and acquired prosopagnosia, where a prominent reduction in the structural integrity of both ILF and IFOF bilaterally was evident [36,37]. Parietal connections, particularly with the Superior Parietal Lobule (SPL), were initially absent in earlier studies but have emerged with stronger evidence recently. However, marginal projections to areas like the superior occipital gyrus, calcarine cortex, and occipital pole indicate inconsistency and variability in the identified terminations.
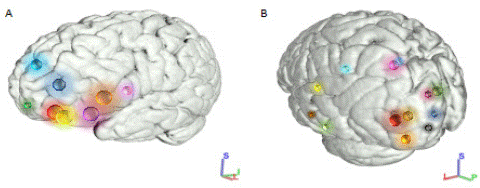
Figure 2: Schematic representation of the cortical distributions of the IFOF. 3D representation Freesurfer-based regions, (left lateral view).
A. Anterior distributions of cortical connections. Light green: frontal pole; yellow and red: lateral and medial portions of the orbitofrontal cortex; purple: pars orbitalis; orange: pars triangularis; pink: pars opercularis of inferior frontal cortex; blue: middle frontal cortex; light blue: superior frontal cortex.
B. Posterior distributions of cortical connections. Red: projection of lingual gyrus; orange: inferior occipital gyrus; gold: middle occipital gyrus; green: projection of cuneus; pink: superior parietal lobule; light green: projection of fusiform gyrus; purple: superior occipital gyrus; black: occipital pole; blue: calcarine; aquamarine: angular gyrus; light blue: precuneus; yellow: superior temporal gyrus; grey: middle temporal gyrus; brown: inferior temporal gyrus.
The limited consensus and variability in findings across studies raise concerns about the accuracy and reliability of identifying specific cortical connections of the IFOF. The discrepancies might arise from methodological differences, including variations in dissection techniques, DTI applications, and tractography methodologies. Additionally, the lack of consistent identification across multiple studies for certain cortical regions emphasizes the complexity and variability in the IFOF's anatomical connectivity. This underscores the need for further research using standardized methodologies to better define and understand the cortical distributions of the IFOF's connections, ensuring more accurate and reliable findings.
References
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press. 2006.
- Burdach KF. Vom Baue und Leben des Gehirns. Leipzig: Dyk. 1822.
- Dejerine J. Anatomie des Centres Nerveux. Paris: Rueff et Cie. 1895.
- Trolard JB. Le faisceau longitudinal inferieur du cerveau. Revue Neurologique. 1906; 14: 440e446.
- Curran E. A new association fibre tract in the cerebrum. With remarks of the fibre tract dissection method of stud-ying the brain. Journal of Comparative Neurology and Psychology. 1909; 19: 1e18.
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fascicu-lus revisited in the lights of brain stimulation data. Cortex. 2010; 46: 691–699.
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013; 218: 21–37.
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002; 17: 77-94.
- Mori S and van Zijl PC. Fiber tracking: Principles and strategies - a technical review. NMR in Biomedicine. 2002; 15(7e8): 468-480.
- Mori S, Wakana S, Nagae-Poetscher LM, and Van Zijl PCM. MRI Atlas of Human White Matter. Amsterdam: Elsevier. 2005.
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibres pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007; 130: 630-653.
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012; 48: 82-96.
- Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One. 2014; 9: e100274.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015; 350: g7647
- Reil JC. Die Sylvische Grube oder das Thal, das gestreifte grosse Hirnganglium, dessen Kapsel und die Seitentheile des grossen Gehirns. Archiv fur die Physiologie. 1809; 9: 195e208.
- Charpy A. Syste`me nerveux (encephale). Paris: Masson et Cie, Editeurs libraires de l’academiede medicine. 1895.
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell’acqua F, Danek A, et al. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2012.
- Mars RB, Foxley S, Verhagen L, Jbabdi S, Sallet J, Noonan MP, et al. The extreme capsule fiber complex in hu-mans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Struct Funct. 2016; 221: 4059–4071.
- Sarubbo S, Petit L, De Benedictis A, Chioffi F, Ptito M, Dyrby TB. Uncovering the inferior fronto-occipital fascicle and its topological organization in non-human primates: the missing connection for language evolution. Brain Struct. Funct. 2019; 224: 1553–1567.
- Rudrauf D, David O, Lachaux J-P, Kovach CK, Martinerie J, Renault B, et al. Rapid interactions between the ventral visual stream and emotion-related structures rely on a two- pathway architecture. Journal of Neuroscience. 2008; 28: 2793-2803.
- ffytche DH and Catani M. Beyond localization: From hodology to function. Philosophical Transaction of the Royal Society of London. 2005; 360: 767-779.
- Kawasaki H, Kaufman O, Damasio H, Damasio AR, Granner M, Bakken H, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience. 2001; 4: 15-16.
- Fernandez-Miranda JC, Rhoton AL Jr, Alvarez-Linera J, Kakizawa Y, Choi C, de Oliveira EP. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008; 62: 989–1026.
- Martino J, Vergani F, Robles SG, Duffau H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotem-poral and temporoinsular structures. Neurosurgery. 2010; 66: 4-12.
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005; 128: 797–810.
- Wu Y, Sun D, Wang Y, Wang Y. Subcomponents and Connectivity of the Inferior Fronto-Occipital Fasciculus Re-vealed by Diffusion Spectrum Imaging Fiber Tracking. Front Neuroanat. 2016; 10: 88.
- Panesar SS, Yeh FC, Deibert CP, Fernandes-Cabral D, Rowthu V, Celtikci P, et al. A diffusion spectrum imaging-based tractographic study into the anatomical subdivision and cortical connectivity of the ventral external capsule: uncinate and inferior fronto-occipital fascicles. Neuroradiology. 2017; 59: 971-987.
- Hau J, Sarubbo S, Perchey G, Crivello F, Zago L, Mellet E, et al. Cortical Terminations of the Inferior Fronto-Occipital and Uncinate Fasciculi: Anatomical Stem-Based Virtual Dissection. Front Neuroanat. 2016; 10: 58.
- Motomura K, Fujii M, Maesawa S, Kuramitsu S, Natsume A, Wakabayashi T. Association of dorsal inferior frontooccipital fasciculus fibers in the deep parietal lobe with both reading and writing processes: a brain mapping study. J Neurosurg. 2014; 121: 142-8.
- Herbet G, Yordanova YN, Duffau H. Left Spatial Neglect Evoked by Electrostimulation of the Right Inferior Fronto-occipital Fasciculus. Brain Topogr. 2017; 30: 747–756.
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell’Acqua F, Allin M, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractog-raphy. Neuroimage. 2011; 54: 49-59.
- Conner AK, Briggs RG, Sali G, Rahimi M, Baker CM, Burks JD, et al. A Connectomic Atlas of the Human Cere-brum-Chapter 13: Tractographic Description of the Inferior Fronto-Occipital Fasciculus. Oper Neurosurg (Hager-stown). 2018; 15: S436-S443.
- Duffau H. Awake Surgery for Left Posterior Insular Low-Grade Glioma Through the Parietorolandic Operculum: The Need to Preserve the Functional Connectivity. A Case Series. Front Surg. 2022; 8: 824003.
- Kanwisher N, Mcdermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997; 17: 4302–431.
- Unger A, Alm KH, Collins JA, O’Leary JM, Olson IR. Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. Journal of the International Neuropsychological Society. 2016; 22: 180-190.
- Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009; 12: 29-31.
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience. 2009; 29: 15089-15099.