
Research Article
Austin Neurosurg Open Access.2015;2(1): 1027.
Supine Position for the Prevention of Brain Shift in DBS Surgery: Technical Note and Novel Hypothesis “Water in the Inverted Cup” Mechanism
Miyagi Y1,2*, Samura K3, Kishimoto J4 and Chen X5
1Department of Stereotactic and Functional Neurosurgery, Kaizuka Hospital, Japan
2Department of Clinical Neurophysiology, Faculty of Medical Sciences, Kyushu University, Japan
3Department of Neurosurgery, Fukuoka University Hospital, Japan
4Center for Clinical and Translational Research, Kyushu University Hospital, Japan
5Department of Mechanical Engineering, Faculty of Engineering, Yamaguchi University, Japan
*Corresponding author: Miyagi Y, Department of Stereotactic and Functional Neurosurgery, Kaizuka Hospital, 7-7-27 Hakozaki, Higashi-ku, Fukuoka, 812-0053 Japan
Received: May 04, 2015; Accepted: June 11, 2015; Published: June 15, 2015
Abstract
Many neurosurgeons perform DBS implantation in semi-sitting position and perforate a burr hole at the top of the cranium. We have empirically found that the supine position is the best position to minimize CSF leakage, intracranial air invasion and brain shift. The dynamics of brain shift can be explained by the “water in the inverted cup” hypothesis and the significance of the simultaneous fulfillment of three conditions: 1) supine position to minimize negative intracranial pressure; 2) arachnoid sealing to maximize surface tension of CSF; and 3) lower burr hole level to keep the balance of intra/extracranial pressures. Although 1) and 3) sound totally contradictory to conventional ideas, a simple but novel hypothesis the “water in the inverted cup” mechanism successfully explains the dynamics of CSF and air, the brain shift and the phenomena related to various procedures during stereotactic surgery.
Keywords: Brain shift; Supine position; Deep brain stimulation; Stereotactic neurosurgery; Intracranial pressure; Cerebrospinal fluid; Pneumocephalus; Surface tension
Abbreviations
DBS: Deep Brain Stimulation; CSF: Cerebrospinal Fluid; MER: Microelectrode Recording; MR: Magnetic Resonance; AC: Anterior Commissure; PC: Posterior Commissure; CT: Computerized Tomography
Introduction
Brain shift, which affects the clinical accuracy of a neuronavigation system in open craniotomy [1], has been recognized as one of significant factors which introduce error in DBS surgery as well [2-8]. Because brain shift is associated with outflow of CSF and intracranial air invasion, past research has indicated the significance of preventing CSF outflow using a head-up position [3,4,7-12] or arachnoid sealing around an inserted cannula and microelectrode [9,10,13-15]. Since we found that a brain shift occurs due to intracranial air invasion alone, even without significant CSF outflow [7], we have performed DBS surgeries at various angles of head elevation (ranging from supine to semi-sitting position) in order to find the optimal angle which would minimize the brain shift, and we have empirically recognized the significance of the simultaneous fulfillment of three conditions: supine position, arachnoid sealing, and burr-hole perforation around coronal suture. We report our findings of brain shift using these techniques and describe the details of two representative cases (the case with the largest brain shift in this series and the case with marked brain atrophy and large arachnoid cysts). The dynamics of brain shift during surgery are well explained by the “water in the inverted cup” phenomenon.
Methods
The three important points in this procedure were 1) supine position, 2) the burr hole perforation at the coronal suture level, and 3) minimal arachnoid penetration and arachnoid sealing.
The patient wore a stereotactic frame (Leksell model G, Elekta) on the head after local infiltration with 1% lidocaine hydrochloride. The frame was secured perpendicular to the facial plane (including forehead and bilateral zygomatic processes). After the localizer box was mounted on the head, 1.5-T MR images were obtained (Achieva 1.5T SE; PHILIPS). A 3D multiplanar T1-weighted scan (145 slices; voxel size 1.0 x 1.0 x 2.0 mm; TR 25.0 msec; TE 4.6 msec) and a T2- weighted coronal scan (40 slices; voxel size 0.0.53 x 0.53 x 2.0 mm; TR 2000 msec; TE 131 msec) were obtained. The head was secured to the frame holder of the operative table and the patient was placed in a completely supine position without any head flexion. Using stereotactic planning software (Leksell SurgiPlan® ver. 10.1.1, Elekta), the 3D coordinates of the AC and PC, bilateral targets and the stereotactic trajectory were determined.
The entry point was placed within 1cm of the coronal suture. Under a local infiltration with 1% lidocaine hydrochloride, the curved skin incision and dual-floor burr hole (14 mm) [16] was perforated at the entry point, and the dura mater was cauterized using a bipolar coagulator and cut in cruciform within 5 mm in diameter. After mounting two BenGun cannulas with multi-channel microelectrodes on the stereotactic arc, the pia mater was perforated with the 5mm tip of microelectrodes on the avascular point of the gyral surface, and the outer cannula was advanced ahead of the microelectrode tip. After setting two-channel cannulas with microelectrodes, the arachnoid around the penetration points was carefully sealed with fibrin glue to prevent CSF leakage. The MER was performed along two tracks 2 mm apart; first, central and lateral tracks for the right MER, followed by the second central and posterior tracks for the left MER. After the physiological localization of the target by both MER and macrostimulation methods [17], a DBS lead (model 3387 for pallidal simulation and model 3389 for subthalamic stimulation, Medtronic, Inc.) was inserted and anchored with the standard bur hole caps included in DBS kits [18]. With each procedure, the site and direction of the tip of the microelectrodes and DBS leads, as well as the intracranial air, were monitored with stereotactic X-ray films. The distal end of the lead was introduced subcutaneously to the parietal region by a tunneling tool and was embedded under the scalp. The subcutis of the incision was closed using a buried continuous suture with an absorbable suture, and the skin was closed with 4-0 Nylon.
Soon after the surgery, the patient was transferred to the CT and MR imaging units while being kept in a supine position. The postoperative 3D T1-weighted MR images were reconstructed and co-registered with the preoperative 3D images using Leksell SurgiPlan® software [19]. The information of lead location in each patient was utilized for contact selection and parameter setting in the postoperative DBS management. A neurostimulator with its extension cable was implanted under a general anesthesia on the second day postoperatively. The differences in 3D coordinates (ΔX, ΔY and ΔZ) of AC and PC were measured to directly analyze the brain shift. All values were expressed as the mean ± SD in the text, and the Box-Whisker graph (median, 1st and 3rd quartiles, lowest and highest) was used in Figure 1.
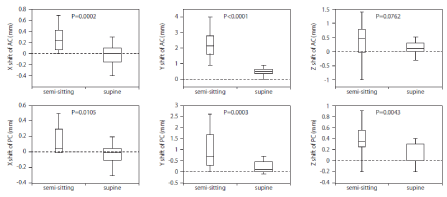
Figure 1: Box-Whisker graph showing the extent of shifts in AC and PC in each axis. The box represents the distance between the 1st and 3rd quartiles. The line
in the box represents the median. The whiskers show the lowest and highest data points. The data of the brain shift during the semi-sitting position were from our
previous report [7]. Positive values denote left, posterior, and inferior shifts along the x, y and z axes, respectively. P values were calculated by Student’s t-test
when the previous data [7] was assumed to be a control. AC= Anterior Commissure; PC= Posterior Commissure.
Results
From October 2011 to May 2013, 28 patients (15 males and 13 females) underwent the contemporaneous implantation of bilateral DBS electrodes at 6 globus pallidus internus for dystonia and 22 subthalamic nuclei for Parkinson’s disease at Kaizuka Hospital. The mean age at the time of surgery was 58.0 ± 12.0 years (range, 21-81 years). The total operation time was 198.6 ± 16.0 minutes (range, 166- 235 min). No patient presented with perioperative complications, such as cerebral hemorrhage or pulmonary air embolism. Brain shift was assessed as changes in the 3D coordinates of AC and PC with MR imaging before and immediately after the implantation surgery.
In the supine position, the shifts of AC were as follows: -0.02 ± 0.16 mm laterally (ΔX), 0.48 ± 0.29 mm posteriorly (ΔY), 0.11 ± 0.19 mm ventrally (ΔZ). The shifts of PC were as follows: -0.02 ± 0.13 mm laterally (ΔX), 0.24 ± 0.25 mm posteriorly (ΔY), 0.11 ± 0.18 mm ventrally (ΔZ). In supine position, the maximum shift (up to 1.0 mm) was observed in posterior shift of AC, and all other shifts of AC and PC in the supine position were less than 1.0 mm. As a reference, our previous data of semi-sitting position and twist-drill surgery performed from December 2000 to July 2001 [7] were also shown in Figure 1.
Case 1 (Dystonia case with the largest posterior brain shift)
A 44-year-old male with cervical dystonia underwent a pallidal stimulation. The AC-PC line was 24.8 mm and the third ventricle width was 6 mm. His intraoperative X-ray film showed a small amount of intracranial air at the frontal top of the cranium, which was much higher than the burr hole level (Figure 2A). MR and CT images immediately after the surgery showed a modest air invasion (Figure 2B and 2C), and the AC shift was 1.0 mm in the posterior direction. His postoperative course was uneventful, and the cervical dystonia was remarkably alleviated at the two-month follow-up visit. There was neither curving of pallidal leads nor residual air on X-ray image after a week (Figure 2D).
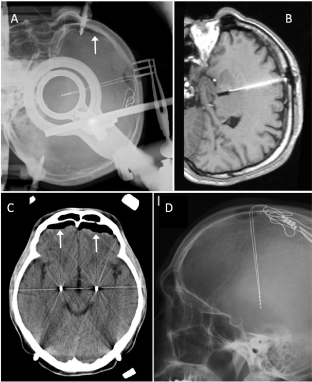
Figure 2: Perioperative neuroimages of a dystonia case which showed the
maximal posterior shift of AC (1.0mm). A: A stereotactic X-ray film of lateral
projection just after anchoring the second DBS lead in contemporaneous
bilateral surgery. An arrow indicates intracranial air. B: A sagittal T1-weighted
MR image including the pallidal lead. C: A transverse CT section including
the thickest point of the intracranial air. B and C were obtained just after
leaving the operating room. D: An X-ray film (non-stereotactic) at one week
postoperatively.
Case 2 (Parkinson’s disease with large arachnoid cysts in bilateral temporal lobe)
A 66-year-old woman had Parkinson’s disease (Hoehn-Yahr stage 3) with wearing off and an exhausting peak-dose dyskinesia. Preoperative MR images showed an enlarged subdural space due to brain atrophy and large arachnoid cysts in bilateral temporal lobes (Figure 3A and 3B). The AC-PC line was 22.0 mm, and the third ventricle width was 6.0 mm. Intraoperative X-P monitoring showed a tiny air bubble underneath the frontal bone (Figure 3C), and postoperative CT showed a very small amount of intracranial air (Figure 3D). The posterior shift of AC was only 0.4 mm, and the shifts in the other directions were less than 0.1 mm. Her postoperative course was uneventful, and the motor fluctuation was successfully abolished.
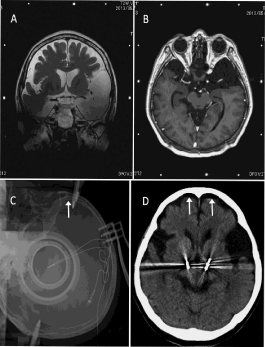
Figure 3: Perioperative neuroimages of a case of Parkinson’s disease with
marked brain atrophy and large arachnoid cysts in bilateral temporal lobe.
The AC shift was only 0.4mm in the posterior shift. A and B: The preoperative
MR images for stereotactic planning: T2-weighted coronal (A) and T1-
weighted axial (B) sections. C: Stereotactic X-ray film of lateral projection just
after anchoring the second DBS lead in contemporaneous bilateral surgery.
D: A transverse CT section including the thickest point of the intracranial
air accumulation just after leaving the operating room. Arrows indicate
intracranial air.
Discussion
Brain shift has been recognized as one of the significant error factors which introduce error during stereotactic functional neurosurgery [5,7,8,12] since the first reported X-ray study by Gerdes et al. [20]. Several perioperative factors that affect brain shift have been proposed: cerebral atrophy [8,21], the number of MER tracks [21], operation time [9,12,22], and staged or non-staged bilateral procedure [13]. Although the extent of brain shift has been shown to relate to intracranial air volume, the linear relationships were not strong enough to quantitatively evaluate a brain shift [3,21]. Therefore, a brain shift in DBS implantation should be quantified directly by the coordinates of the deep brain structure, such as AC and PC, but not by the postoperative intracranial air volume. By directly measuring brain shift, we have detected only small brain shifts (less than 1 mm even in the posterior direction), which would not affect stereotactic accuracy in DBS surgery performed on patients in the supine position. The importance of the supine position during the operative procedure is also suggested by other groups in reproducing the conditions identical to neuroimaging studies used for stereotactic planning, as well as preventing intracranial air invasion and venous air embolism, but not affecting the risk of hemorrhagic complication [13,14].
In a previous report, we used the method of stereotactic cranial perforation by twist drill in the semi-sitting position in order to prevent CSF outflow, and there was no evidence of efficacy of head elevation (or the semi-sitting position of ca. 40˚) with twist drill surgery [23]. The DBS implantation surgery in the semi-sitting position produced 2.33 ± 0.93 (max. 4.0) mm posterior shift of AC even in the absence of CSF outflow; therefore, the negative intracranial pressure in the semi-sitting position and the decrease in brain volume due to increased venous return were considered to facilitate the intracranial air invasion [7]. Since 2007, we have sought the optimal positioning for stereotactic DBS lead implantation. Contrary to our previous expectation, the supine position has been empirically the best condition for preventing brain shift in association with the use of arachnoid sealant.
One may be concerned that CSF located above the burr hole level would easily flow out when the burr hole is perforated as low as the coronal suture in the supine position. However, the phenomenon can be compared to the “water in the inverted cup,” a simple science experiment that is as follows: Fill a cup with water to the top, place a note card over the top of the cup, quickly turn the cup upside down, holding the note card in place and then carefully release the note card. As long as the note card is parallel to the ground, the note card will stay in place and water will not fall out because the atmospheric pressure pushes the card evenly against the water weight. Furthermore, the surface tension of the water molecules prevents the break of the water surface to keep the air outside, even if a small pinhole is made in the card or a slit opens between the card and the cup (Figure 4A). This situation explains why CSF does not come out of the cranial window made around the coronal suture level in the supine position. If one pierces the cup at a point near the water level (Figure 4B), the balance between gravity pulling on the water in the inverted cup and the surface tension of the water will determine whether an air bubble comes inside, and in many instances, the water and the card will fall down. Similarly, if there is some head elevation, the intracranial pressure at the level of CSF channel (burr hole) decreases to nearly atmospheric pressure, making it easier for an air bubble to come inside (and for CSF to go outside), overcoming the surface tension of CSF. If one pierces the bottom of the cup, negative pressure made by the water weight is lost immediately, and the water and card fall down. Similarly, if there is a considerable head elevation angle or a cranial perforation is made close to the top of the calvarium in order to avoid CSF leakage [7], the intra/extracranial pressure difference will be lost easily (Figure 4C), resulting in marked brain shift due to intracranial air invasion. Furthermore, if there is too much air invasion, the weight of the frontal lobe above CSF level presses the brain itself, changes CSF distribution (from ventricle to subarachnoid space) and deforms its shape. Namely, a supine position was found to be optimal for creating the pressure difference to avoid both the CSF leakage and the intracranial air invasion. Even in a supine position, the burr hole opening at the level of the coronal suture does not let CSF go out for a short time if the CSF channel of arachnoid is small enough to keep the surface tension by being adequately sealed with fibrin glue, melted bone wax, or another sealant.
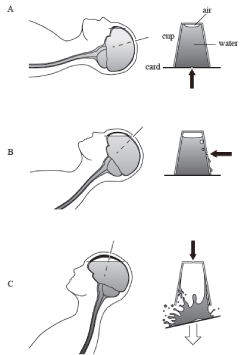
Figure 4: Schematic drawings showing the intraoperative brain shift in each
position. A: Complete supine position. B: Supine with head elevation (or semisitting
with a low angle). C: Semi-sitting position (high angle) and the cranial
perforation at the highest point. The illustrations on the right side explain the
dynamics of brain shift in each position using the “water in the inverted cup”
phenomenon. Arrows indicate the points of small perforation corresponding
to burr hole, dural opening, and arachnoid penetration.
Another presumable mechanism is the positional shift of the brain itself [7]. By the analysis of MR, the brain and spinal cord are known to shift in the skull and spinal canal in a gravity-dependent manner, respectively [24,25]. Even without surgical intervention, it is suggested that the time course of physiological brain shift was within 12 minutes (measuring the time of the 3D MR scan) at the least [25] and that of the spinal cord was 14 seconds (duration of breath holding) at the least [24]. Therefore, the brain is thought to shift promptly, more caudally in the semi-sitting position and more posteriorly in the supine position in physiological condition. While the caudal shift makes frontal subdural space wider (Figure 4C), the posterior shift in the supine position makes the frontal subdural space around the burr hole narrower and the subdural space and CSF channel may be reduced or sealed from inside by the brain itself in the supine position (Figure 4A). Azmi et al. [21] found that total intracranial air volume after surgery correlated with the degree of cerebral atrophy, namely the ratio of extra axial CSF to total intracranial volume, but not the ratio of ventricular volume. Nazzaro et al. [14] also suggested the significance of brain entry via a limited dural opening and involving a gyrus very close to the inner skull. These implications are as important as arachnoid sealing in a sense that the CSF channel should be minimized to keep the surface tension of CSF and the balance of intracranial and atmospheric pressures.
Many studies detected no relationship between the operation time (duration from dural opening to closure) and the extent of brain shift [3-5,7,9] and we agree with the speculation by Coenen et al. that most of the CSF loss or air invasion occurs during the initial moments after penetrating the arachnoid [9]. We also speculate the volume of CSF loss (or intracranial air volume) may depend on the equilibrium between the surface tension of CSF and the difference of intra/extracranial pressures. In many instances, the complete sealing of the arachnoid is impossible and some amount of CSF (or air) will go out (or come in) until equilibrium is reached. If the CSF channels are too large, the surface tension of CSF will be easily broken by air invasion and CSF outflow, lowering the CSF level down to the burr hole level with a marked brain shift. In case 2, however, the brain shift was successfully minimized even in the presence of enlarged subdural space or large arachnoid cysts of bilateral temporal regions (Figure 3), which suggested that the CSF channel successfully minimized by arachnoid sealing maintained the surface tension of CSF and the difference of intra/extracranial pressures at the burr hole attenuated CSF outflow.
Since we have empirically concluded an apparent effectiveness of supine position in DBS surgery, we could not introduce semi-sitting position again even for the sake of a randomized controlled trial from an ethical point of view. However, this hypothesis would need a randomized controlled study to be widely accepted.
Conclusion
The mechanism of the “water in the inverted cup” phenomenon explains the dynamics of brain shift and the importance of the combination of three simple techniques: supine position, cranial perforation close to the coronal suture, and arachnoid sealing, which help to maintain the difference between the intracranial and atmospheric pressures and minimize the brain shift by simultaneously attenuating CSF outflow and intracranial air invasion in DBS surgery.
Disclosure Statement
This study was partly supported by Grant-in-Aid for Scientific Research (B) JSPS Kakenhi (24390345), Japan and Grant-in-Aid from the Ministry of Health, Labor and Welfare (201024171A). A part of this work was presented in flash presentation at the 16th quadrennial meeting of the World Society of Stereotactic and Functional Neurosurgery (WSSFN), Tokyo, Japan, May 29, 2013. We greatly appreciate Hiroshi Takahashi M.D., Ph.D., Department of Neurosurgery, Tokyo Metropolitan Neurological Hospital for his helpful comment on this study. We also appreciate Ms. Nancy Venarske and Dr. Daniel Venarske for their assistance in preparing this manuscript.
References
- Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000; 47: 1070-1079.
- Derrey S, Lefaucheur R, Proust F, Chastan N, Leveque S, Gerardin E, et al. The unsuccessful placement of a deep brain stimulation electrode due to a brain shift induced by air invasion: Case report. Parkinsonism Relat Disord. 2011; 17: 393-394.
- Elias WJ, Fu KM, Frysinger RC. Cortical and subcortical brain shift during stereotactic procedures. J Neurosurg. 2007; 107: 983-988.
- Halpern CH, Danish SF, Baltuch GH, Jaggi JL. Brain shift during deep brain stimulation surgery for parkinson's disease. Stereot Funct Neurosurg. 2008; 86: 37-43.
- Khan MF, Mewes K, Gross RE, Skrinjar O. Assessment of brain shift related to deep brain stimulation surgery. Stereotact Funct Neurosurg. 2008; 86: 44-53.
- Kramer DR, Halpern CH, Danish SF, Jaggi JL, Baltuch GH. The effect of intraventricular trajectory on brain shift in deep brain stimulation. Stereotact Funct Neurosurg. 2012; 90: 20-24.
- Miyagi Y, Shima F, Sasaki T. Brain shift: An error factor during implantation of deep brain stimulation electrodes. J Neurosurg. 2007; 107: 989-997.
- Obuchi T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, Yamamoto T. Direction and predictive factors for the shift of brain structure during deep brain stimulation electrode implantation for advanced parkinson's disease. Neuromodulation. 2008; 11: 302-310.
- Coenen VA, Abdel-Rahman A, McMaster J, Bogod N, Honey CR. Minimizing brain shift during functional neurosurgical procedures - a simple burr hole technique that can decrease csf loss and intracranial air. Cent Eur Neurosurg. 2011; 72: 181-185.
- Takumi I, Mishina M, Hironaka K, Oyama K, Yamada A, Adachi K, et al. Simple solution for preventing cerebrospinal fluid loss and brain shift during multitrack deep brain stimulation surgery in the semisupine position: Polyethylene glycol hydrogel dural sealant capping. Neurol Med Chir (Tokyo). 2013; 53: 1-6.
- van den Munckhof P, Contarino MF, Bour LJ, Speelman JD, de Bie RM, Schuurman PR. Postoperative curving and upward displacement of deep brain stimulation electrodes caused by brain shift. Neurosurgery. 2010; 67: 49-53.
- Winkler D, Tittgemeyer M, Schwarz J, Preul C, Strecker K, Meixensberger J. The first evaluation of brain shift during functional neurosurgery by deformation field analysis. J Neurol Neurosurg Psychiatry. 2005; 76: 1161-1163.
- Hunsche S, Sauner D, Maarouf M, Poggenborg J, Lackner K, Sturm V, et al. Intraoperative x-ray detection and mri-based quantification of brain shift effects subsequent to implantation of the first electrode in bilateral implantation of deep brain stimulation electrodes. Stereotact Funct Neurosurg. 2009; 87: 322-329.
- Nazzaro JM, Lyons KE, Honea RA, Mayo MS, Cook-Wiens G, Harsha A, et al. Head positioning and risk of pneumocephalus, air embolism, and hemorrhage during subthalamic deep brain stimulation surgery. Acta Neurochir (Wien). 2010; 152: 2047-2052.
- Slotty PJ, Kamp MA, Wille C, Kinfe TM, Steiger HJ, Vesper J. The impact of brain shift in deep brain stimulation surgery: Observation and obviation. Acta Neurochir (Wien). 2012; 154: 2063-2068.
- Yamamoto T, Katayama Y, Kobayashi K, Oshima H, Fukaya C. Dual-floor burr hole adjusted to burr-hole ring and cap for implantation of stimulation electrodes. Technical note. J Neurosurg. 2003; 99: 783-784.
- Miyagi Y, Okamoto T, Morioka T, Tobimatsu S, Nakanishi Y, Aihara K, et al. Spectral analysis of field potential recordings by deep brain stimulation electrode for localization of subthalamic nucleus in patients with parkinson's disease. Stereotact Funct Neurosurg. 2009; 87: 211-218.
- Samura K, Miyagi Y, Okamoto T, Hayami T, Kishimoto J, Katano M, et al. Short circuit in deep brain stimulation. J Neurosurg. 2012; 117: 955-961.
- Yoshida F, Miyagi Y, Morioka T, Hashiguchi K, Murakami N, Matsumoto K, et al. Assessment of contact location in subthalamic stimulation for parkinson's disease by co-registration of computed tomography images. Stereotact Funct Neurosurg. 2008; 86: 162-166.
- Gerdes FU, Klein G, Nadjmi M, Schaltenbrand G. [X-ray studies of the brain as a basis for stereotaxy (author's transl)]. J Neurol. 1975; 210: 183-190.
- Azmi H, Machado A, Deogaonkar M, Rezai A. Intracranial air correlates with preoperative cerebral atrophy and stereotactic error during bilateral stn dbs surgery for parkinson's disease. Stereotact Funct Neurosurg. 2011; 89: 246-252.
- Petersen EA, Holl EM, Martinez-Torres I, Foltynie T, Limousin P, Hariz MI, et al. Minimizing brain shift in stereotactic functional neurosurgery. Neurosurgery. 2010; 67: ons213-221.
- Miyagi Y, Shima F, Ishido K. Implantation of deep brain stimulation electrodes in unshaved patients. Technical note. J Neurosurg. 2002; 97: 1476-1478.
- Holsheimer J, den Boer JA, Struijk JJ, Rozeboom AR. MR assessment of the normal position of the spinal cord in the spinal canal. AJNR Am J Neuroradiol. 1994; 15: 951-959.
- Schnaudigel S, Preul C, Ugur T, Mentzel HJ, Witte OW, Tittgemeyer M, et al. Positional brain deformation visualized with magnetic resonance morphometry. Neurosurgery. 2010; 66: 376-384.