
Special Article - Brain Aneurysms
Austin Neurosurg Open Access.2015;2(2): 1028.
Treatment of a Ruptured Dissecting Aneurysm of the Proximal Middle Cerebral Artery by Trapping and High-Flow Extracranial-Intracranial Bypass: A Case Report
Ota T¹* and Mizutani T²
1Department of Neurosurgery, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan
2Department of Neurosurgery, School of Medicine, Showa University, Tokyo, Japan
*Corresponding author: Takahiro Ota, Department of Neurosurgery, Tokyo Metropolitan Tama Medical Center, 2-8-29 Musasi-dai, Fuchu, Tokyo 183-8524, Japan
Received: May 25, 2015; Accepted: June 24, 2015; Published: June 26, 2015
Abstract
Background: Dissecting aneurysm (DA) of the proximal Middle Cerebral Artery (MCA) is uncommon, and there are only a few reports of ruptured M1 dissecting aneurysms. The M1 segment, which has numerous Lenticulostriate Arteries (LSAs), is difficult to treat with either direct clipping or endovascular therapy. Our report is the first to describe a ruptured M1 dissecting aneurysm that is successfully treated with trapping and external carotid artery-to-MCA bypass.
Case Description: A 40-year-old woman presented with dissecting aneurysm of the right MCA, manifesting as subarachnoid hemorrhage. Right carotid arteriography revealed aneurysmal dilation of the proximal M1 segment, between the origin of the MCA and the origin of the anterior temporal artery. Rebleeding occurred on Day 4, and repeating the right carotid arteriography indicated enlargement of the M1 lesion and the bleb of the aneurysm. Surgery was performed on Day 5 to devascularize the DA of the M1 segment and revascularize the MCA area. High-flow bypass using saphenous vein grafting occurred prior to aneurysm trapping. Only the most medial of the LSAs was sacrificed and the postoperative course was uneventful. Magnetic resonance imaging revealed a small infarction in the right lenticular nucleus. The patient was discharged without neurological deficits on Day 28.
Conclusion: Trapping with high-flow bypass appears feasible for treatment of ruptured M1DA.
Keywords: Dissecting aneurysm; Subarachnoid hemorrhage; Middle cerebral artery; Trapping; High-flow bypass
Abbreviations
DA: Dissecting Aneurysm; MCA: Middle Cerebral Artery; LSA: Lenticulostriate Artery; ATA: Anterior Temporal Artery; CT: Computed Tomography; MRI: Magnetic Resonance Imaging
Introduction
Dissecting Aneurysm (DA) of the Middle Cerebral Artery (MCA) is uncommon compared to aneurysm of the vertebrobasilar artery. While there are several case reports of patients with DA of the MCA, only a small number of reports have described ruptured dissecting aneurysms of the M1 segment (M1DA) [1-9]. The M1 segment has numerous Lenticulostriate Arteries (LSAs) that are often included in the aneurysmal segment, so DA of the proximal M1 is challenging to treat with both direct clipping and endovascular therapy. Treatment efficacy depends on whether the LSAs were affected, and on the length of the dissection. This is the first report of a rare case of ruptured M1DA successfully being treated using trapping and external carotid artery-to-MCA bypass with interposed saphenous vein grafting.
Case Report
Our patient was a 40-year-old woman admitted with sudden onset of severe headache and vomiting. She had no relevant past medical history and neurological examination at admission was normal. Computed Tomography (CT) indicated subarachnoid hemorrhage (Figure 1a) and an angiographic aneurysmal dilation of the proximal M1, between the origin of the MCA and the origin of the Anterior Temporal Artery (ATA), suggesting dissecting aneurysm (Figure 1b). Neither atherosclerotic changes nor probable sites for saccular aneurysms were detected. Most LSAs were distal from the dissected site, and the majority of LSAs were preserved. However, we were not confident that the origin of the ATA had been dissected, based on angiographic findings. The patient was maintained under mild sedation due to a delay in proceeding with the operation. Rebleeding occurred at the right Sylvian fissure on Day 4, and included seizure (Figure 1c). Right carotid arteriography at that time indicated enlargement of the M1 lesion and revealed the bleb of the aneurysm (Figure 1d). The subarachnoid hemorrhage was hypothesized to originate from the M1DA, so surgery was performed on Day 5 to devascularize the M1DA and revascularize the MCA territory.
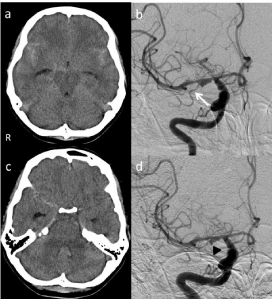
Figure 1: a) CT indicating subarachnoid hemorrhage. b) Right carotid
angiography revealing aneurysmal dilation of the proximal M1 segment.
White arrow, anterior temporal artery. c) CT on Day 4 indicating rebleeding,
particularly at the right Sylvian fissure. d) Subsequent right carotid
arteriography indicating enlargement of the M1 lesion and the bleb of the
aneurysm (black arrowhead).
Operation
High-flow bypass using a saphenous vein graft occurred prior to aneurysm exposure. A red, thin-walled aneurysm was observed at the M1 segment, which appeared to be a pseudolumen. The lesion extended from just distal to the origin of M1 to the origin of the ATA (Figure 2a). Microscopic inspection revealed that most LSAs were distal from the DA and could therefore be preserved. The M1 segment just proximal to the aneurysm and to the ATA (Figure 2b) was occluded by clipping, following verification of graft patency (Figure 2c). Only the most medial of the LSAs was sacrificed during our procedure.
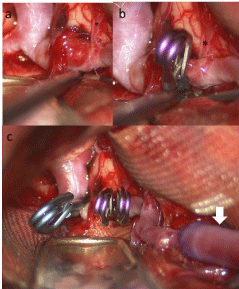
Figure 2: a) M1 segment presenting with a red, thin-walled aneurysm, which
appears to be a pseudolumen. b) A clip was applied just proximal to the origin
of the anterior temporal artery (*). c) Microscopic whole view. The aneurysm
was trapped with 3 clips after high-flow bypass using a saphenous vein graft
(white arrow).
Postoperative course
The postoperative course was uneventful. Postoperative carotid arteriography indicated complete obliteration of the DA and blood flow through the saphenous vein graft to the ATA in a retrograde manner (Figure 3a-3c), along with LSA preservation. Magnetic resonance imaging (MRI) revealed a small infarction in the right lenticular nucleus (Figure 3d). The patient was discharged without neurological deficits on Day 28.
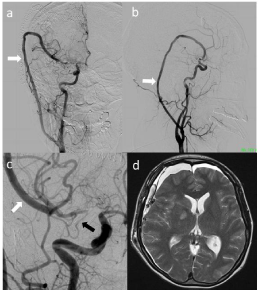
Figure 3: Postoperative angiography showing the patent saphenous vein
graft and right MCA perfusion through the graft (a: AP view; b: lateral view).
c) Magnified view of A. Retrograde blood flow through the saphenous vein
graft (white arrow) of the MCA, which flows to the anterior temporal artery
(black arrow). d) Postoperative MRI showing a small infarction in the right
lenticular nucleus.
Discussion
The incidence of ruptured DA of the MCA is reported as 3.7% (4 of 108 cases) of all ruptured DAs [10]. The proximal MCA (M1), which gives rise to perforating arteries, poses a major challenge for both direct clipping and endovascular therapy. The principal method of treating ruptured DA is to occlude the affected artery, in order to prevent rebleeding. The other important aim of surgical M1DA treatment is to preserve blood flow to the LSAs.
Diagnosis
A number of angiographic characteristics of DAs have been reported, including the string sign, rosette sign, pearl reaction, and double lumen [11]. However, angiography does not always result in definitive diagnosis, because these signs are also observed in atherosclerotic vascular disease. Changes in aneurysmal configuration or rebleeding are highly suggestive of DA, as occurred in this case [2,11]. Therefore, when DA is suspected, diagnostic examinations and serial angiographic studies should be performed immediately, due to the possibility of dynamic changes over a short time.
Operation
Reported cases of M1DA with subarachnoid hemorrhage have had poor patient outcomes in the absence of surgical procedures. Given the high rate of rebleeding and poor natural progression [3,5,12,13]. DA should be treated aggressively. Treatment of M1DA is limited by the M1 perforators, so interventions have included wrapping [2,3,8] or coating the aneurysm, or sometimes direct clipping [7]. However, the reliability of wrapping and coating is unknown, particularly for subarachnoid hemorrhage. Bypass with aneurysmal trapping is one treatment option for M1DA, but trapping of the M1DA without revascularization carries unpreventable risks of cerebral ischemia. Occlusion of the M1DA has 3 possible presentations: proximal to the aneurysm, distal to the aneurysm, or both. Microscopic inspection is the most reliable way to determine the extent of dissection and choose the occlusion site on the affected artery.
Microscopic manipulation in the area surrounding the arterial dissection presents with a risk of rebleeding, so revascularization should be prepared in advance. Treatment by proximal occlusion of the MCA and use of a superficial temporal artery-MCA bypass has resulted in poor patient outcomes [4]. However, high-flow bypass for treatment of M1DA was not previously reported. Accurate evaluation of the collateral circulation within the MCA territory is difficult, but our findings suggest revascularization with high-flow bypass for trapping a ruptured M1DA as an effective therapeutic strategy.
M1DA is rare; nonetheless, it should be considered as a cause of subarachnoid hemorrhage. Treatment must be individualized to patients according to the specific anatomical findings. The LSAs are divisible into medial, intermediate, and lateral groups. Sacrifice of the most medial LSA is less likely to cause ischemic complications, as observed in our patient [14]. Success with trapping a proximal M1DA also depends on whether flow through the high-flow bypass to the LSAs is sufficiently preserved. Therefore, high-flow bypass with trapping is a successful option under the following conditions: aneurysm at the most proximal M1; most LSAs originate distal to the aneurysm; and, most importantly, a branch of the MCA (such as the ATA) is proximal to the LSAs and sufficiently allows retrograde flow from the graft. Under these conditions, high-flow bypass with trapping is a viable treatment option for ruptured M1DA.
Conclusion
Aggressive treatment of ruptured M1DA, using trapping with high-flow bypass, is a valid and useful treatment option.
References
- Kunze S, Schiefer W. Angiographic demonstration of a dissecting aneurysm of the middle cerebral artery. Neuroradiology. 1971; 2: 201-206.
- Niikawa S, Yamada J, Sumi Y, Yamakawa H. Dissecting aneurysm of the middle cerebral artery manifesting as subarachnoid hemorrhage and hemorrhagic infarctions--case report. Neurol Med Chir (Tokyo). 2002; 42: 62-66.
- Mizutani T. Subarachnoid hemorrhage associated with angiographic "stenotic" or "occlusive" lesions in the carotid circulation. Surg Neurol. 1998; 49: 495-503.
- Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Subarachnoid hemorrhage from intracranial dissecting aneurysms of the anterior circulation. Two case reports. Neurol Med Chir (Tokyo). 1999; 39: 442-446.
- Ohkuma H, Suzuki S, Kikkawa T, Shimamura N. Neuroradiologic and clinical features of arterial dissection of the anterior cerebral artery. AJNR American journal of neuroradiology. 2003; 24: 691-699.
- Bosch J, Mauleon A, Coscojuela P, Porta I, Grivé E, Alvarez-Sabín J, et al. [Intraventricular hemorrhage due to the rupture of atherosclerotic dissecting aneurysm of the middle cerebral artery]. Revista de neurologia. 1999; 28: 973-975.
- Isono M, Abe T, Goda M, Ishii K, Kobayashi H. Middle cerebral artery dissecting aneurysm causing intracerebral hemorrhage 4 years after the non-hemorrhagic onset: a case report. Surg Neurol. 2002; 57: 346-349.
- Ning L, Kato Y, Sano H, Nair RB, Yoneda M, Watanabe S, et al. Spontaneous dissecting aneurysm of middle cerebral artery: a case report with review of the literature. Minimally invasive neurosurgery. 2003; 46: 357-360.
- Chuang MJ, Lu CH, Cheng MH. Management of middle cerebral artery dissecting aneurysm. Asian J Surg. 2012; 35: 42-48.
- Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011; 114: 1037-1044.
- Mizutani T. Middle cerebral artery dissecting aneurysm with persistent patent pseudolumen. Case report. J Neurosurg. 1996; 84: 267-268.
- Sasaki O, Koike T, Tanaka R, Ogawa H. Subarachnoid hemorrhage from a dissecting aneurysm of the middle cerebral artery. Case report. J Neurosurg. 1991; 74: 504-507.
- Ohkuma H, Suzuki S, Ogane K. Dissecting aneurysms of intracranial carotid circulation. Stroke. 2002; 33: 941-947.
- Rhoton AL Jr. The supratentorial arteries. Neurosurgery. 2002; 51: S53-120.