
Review Article
Austin Neurosurg Open Access. 2015;2(2): 1031.
The Role of Transient Receptor Potential Channels in the Pathogenesis of Cerebral Aneurysm Formation
Tomasz Tykocki1*, Anna Kornakiewicz2, Albert Acewicz3 and Bogusław Kostkiewicz4
1Department of Neurosurgery, Institute of Psychiatry and Neurology in Warsaw, Poland
2First Faculty of Medicine, Medical University of Warsaw, Poland
3First Department of Neurology, Institute of Psychiatry and Neurology in Warsaw, Poland
4Department of Neurosurgery, Central Clinical Hospital of the Ministry of Interior, Poland
*Corresponding author: Tomasz Tykocki, Department of Neurosurgery, Institute of Psychiatry and Neurology, Sobieskiego 9, Warsaw 02-957, Poland
Received: May 07, 2015; Accepted: June 28, 2015; Published: July 01, 2015
Abstract
Background: Discovery of Transient Receptor Potential (TRP) channels has allowed for a new insight into many issues and doubts concerning the pathogenesis of many diseases including those localized in the cerebrovascular system.
Results: Super family of TRP channels has an impact on the functioning of the cerebral vessels, especially on mechanotransduction and modulation of the fluid flow forces acting on the endothelium. TRP channels modulate endothelial response to prolonged abnormal hemodynamic conditions. They transduce humoral or cell-mediated inflammatory reactions, macrophage infiltration in the arterial wall in the high oxidative stress conditions. In aneurysm formation process smooth muscle cells intensively proliferate and chaotically migrate to the intima causing myointimal hyperplasia. This pathological cascade is controlled by TRP channels. The most documented linkage between aneurysms etiopathogenesis and TRP channels derives from the study on autosomal dominant polycystic kidney disease. Taking into account the expression and function of TRP channels in smooth muscle cells, recent findings highlight the role of TRP channels in the myogenic response in cerebral blood vessels.
Conclusion: TRP channels participate in the stages of cerebral aneurysm formation including mechanotransduction of shear stress, endothelial dysfunction, inflammatory cascade, oxidative stress and structural remodelling of arterial wall.
Keywords: Transient receptor potential; Channels; Aneurysm formation; Cerebral auto regulation
Introduction
The etiopathogenesis of cerebral aneurysms still seems to be mysterious. The hemodynamic conditions during the dynamic flow forces acting on the cerebral vessels modulate endothelial response during non-uniform cerebral blood flow. Long-acting nonphysiological shear stress with extremely high or low shear value could be responsible for the triggering of the pathological cascade of arterial disruption [1,2]. Interestingly, the low shear stress produces arterial wall degeneration by inflammatory response mediating by macrophages and atherosclerosis [3]. Additionally, it has been documented that risk factors of Subarachnoid Haemorrhage (SAH) such as hypertension and smoking play a role in the structural remodeling of the cerebral vessels and precede the aneurysm formation [2]. However, the mechanism initiating a cascade that leads to the formation of an aneurysm is still unclear. The Nordic Twin Study added an intriguing observation on the SAH etiology questioning its genetic origin, reinforcing rather environmental risk factors [4]. The discovery of Transient Receptor Potential (TRP) channels in the late 60’s of the last century has allowed for a new insight into many issues and doubts concerning the pathogenesis of diseases, especially when deliberating the very molecular origin of the biological dysfunction and subsequent cascade leading to the clinical symptoms [5]. Members of TRP super family channels have impact on the functioning of the cerebral vessels, especially on the cerebral auto regulation, transduction and modulation of the fluid flow forces on the endothelium. Finally, TRP channels are probably involved in the pathogenesis of cerebral aneurysm formation, mainly as a trigger in response to prolonged abnormal hemodynamic conditions. The authors discuss the role of TRP channels in the functioning and pathophysiology of the cerebral circulation, starting with the general characteristics, then etiopathogenesis of aneurysm formation.
TRP Channels - General Characteristics and History
Many studies on TRP channels have given insight into the understanding of important molecular pathways and systemic functions, especially those related to the sensorial physiology [6- 8]. The first TRP member was identified in 1969 after the analysis of electroretinograms of Drosophila melanogaster with a transient and abnormal response to light [9]. The TRP gene was cloned in 1989 and found to code a novel amino acid protein which could form a membrane ion channel or function as a receptor. Since 1995, several TRP-related genes have been cloned from mammalian cells which eventually led to the elucidation of more than 50 channels with different properties [10,11]. The mammalian TRP super family consists of 6 subfamilies that are based on sequence homology: TRPC (classical or canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin) and TRPML (mucolipin). Subfamilies are named by the properties of the founding members [12]. According to a primary amino acid sequence homology, mammalian TRP channels can be classified into two groups and further – into six subgroups. Group 1 includes TRPC, TRPV, TRPA and TRPM and group 2 involves TRPP and TRPML.
TRP Channels in the Etiopathogenesis of Cerebral Aneurysms
Hemodynamic forces are crucial determinants in the continuous activation of endothelial cells. They consist of two main vectors: perpendicular to the wall - blood pressure, and parallel to the surface of the endothelium - wall shear stress, [4]. Thus, endothelial cells form a complex mechanical signal-transduction interface between flowing blood and the vessel wall. Hemodynamic forces acting on the cerebral vasculature with special attention to the endothelial wall shear stress might contribute to the formation of aneurysm and trigger its rupture [1,13]. One hypothesis raise that mechanical forces acting on the endothelium are transduced into biologic signals through the activity of TRP channels [14]. The first conclusion that TRP could display mechanosensitive properties came from the study on phenotypes of the mutants for OSM-9 channel in nematode Caenorhabditis elegans. The mutation was shown to be closely linked to the mutations in mammalian TRPV channels [15,16]. Here authors present the current knowledge on the molecular contribution of TRP channels in the cerebral aneurysms formation in the context of the transduction of shear stress induced endothelial response to hemodynamic forces, endothelial dysfunction, inflammatory cascade, oxidative stress and structural remodelling of the arterial wall (Figure 1).
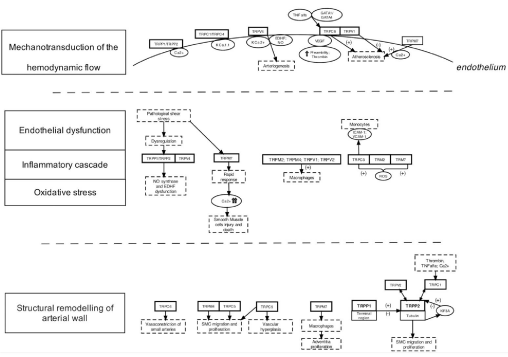
Figure 1: Schematic presentation of the role of transient receptor potential channels in the stages of cerebral aneurysm formation.
TNF- Tumor Necrosis Factor, NO - nitric oxide; EDHF - Endothelium-derived hyperpolarizing factor; VEGF - vascular endothelial growth factor; GATA (globin
transcription factor); ROS - reactive oxygen species; ICAM- intercellular adhesion molecule; VCAM - Vascular cell adhesion protein; (+) – stimulation; (+)- inhibition.
Mechanotransduction of the hemodynamic forces
The shear stress induces increase in Ca2+ concentration in cells of the arterial wall through external mechanosensitive Ca2+- permeable cation channels, which act as mechanosensors in recognizing alterations of hemodynamic forces [17]. Ca2+ sparks are intracellular Ca2+ release events that are important in excitation-contraction coupling and arise from the activation of a cluster of ryanodine receptors. These Ca2+ sparks activate nearby sarcolemmal voltageand Ca2+- activated potassium channels (BK channels) and increase the frequency of macroscopic outward K+ currents [18]. The sheerstress induces oscillations with a complex spatial, temporal and frequency pattern, therefore amplitude of such Ca2+ fluctuations modulates the synthesis of endothelial vasodilators depending on the degree of mechanical stimulation [19]. However, there are several pathways associated with lipid bilayer mechanics, specialized forcetransducing structures, biochemical reactions, membrane trafficking and transcriptional regulation, which have been proposed so far [20]. The suggested mechanisms could be divided into direct and indirect ones. The direct mechanism is based on the membrane model, which presumes direct activation, by alteration in the hemodynamic forces, resulting in tension-dependent stimulus transfer between lipids and transmembrane segments of channels. It has been suggested that TRPC1 channel acts according that model [21,22]. TRPC1 and large conductance calcium-activated, potassium-selective channels (KCa1.1) can form a physical complex in vascular smooth muscle cells and that complex plays an important role in the smooth muscle hyper polarization and control of vascular tone [23]. However, interestingly, alternative model for TRPC channels has also been proposed. There is a study revealing that a mechanosensitive Gq-coupled receptor could induce the downstream molecular pathway of blood vessel myogenic response [24]. Some of TRPC channels have been shown to be paired with and activated by the Gq-PLC signaling system [25]. Previous reports have found that TRPC6 is not a classical mechanosensitive ion channel itself, but is activated by a mechanical stimulus when co-expressed with the angiotensin receptor 1 (AT1R) [24,26]. Agents that inhibit Gq or PLC suppress mechanical activation of the channel. The competitive AT1R antagonist, losartan, inhibits the Bayliss effect, both in cerebral arteries and in isolated perfused kidney [24]. The data has been validated especially for myogenic responsiveness of anterior cerebral arteries [27]. Thus, it has been concluded that mechanical activation of Gprotein coupled receptor might be the missing link between a mechanical stimulus and activation of TRPC channels [26].
The indirect model called distant force-sensing model involves mechanical stimuli transduction through an intracellular signalling cascade, for example through the phospholipase A2 (PLA2) pathway [28]. In this mode, channels are considered mechanically sensitive, but not mechanically gated. This mechanism has been clearly demonstrated in the case ofTRPV4 channel. This channel can be activated directly, by mechanical stimulation of phospholipase A2, or with PLC pathway and membrane G proteins [29-31]. It has been found that shear-stress-induced vasodilation was diminished by Ruthenium Red, suggesting a role for TRPV4 channel in this response [32]. Similar findings have been confirmed by reports demonstrating an absence of shear-stress-induced vasodilation in carotid arteries isolated from TRPV4-knockoutmice [33,34]. Recent studies suggest that mechanical activation of TRPV4 in arteries by physiologically relevant shear stress and flow/reperfusion is a critical component of endothelial mechanotransduction [33].
Shear stress-induced activation of TRPV4 channels might involve tyrosine phosphorylation, as has been suggested for the channel activation by osmotic stress using heterologous expression model [35]. However, inhibition of protein kinase C and tyrosine kinase does not exert any effect, thus it has been concluded that TRPV4 phosphorylation does not play a major role in the shear stress– induced response [36]. It has been found that under conditions of mechanical stress TRPV4 channel induces NO- (nitric oxide) and EDHF- (endothelium-derived hyperpolarizing factor) dependent mechanisms in carotid arteries of rat and mouse [37]. This action could be explained by a functional linkage between TRPV4 and the calcium-dependent potassium channel that would result in endothelial hyper polarization [38].
Activation of TRPP2 or TRPA1 channels leads to the transduction of mechanical forces through the cytoskeleton [39,40]. TRPP1 that links to TRPP2 to build a functional channel may act as a sensory molecule transducing the hemodynamic stimulus to TRPP2, which then triggers Ca2+ sparks [41].
Worth of emphasize is the difference between sheer stress and membrane stretch, because it is not clear whether both activate the same or different channels. There is a study, in which shear stress– activated channel, TRPV4, has been shown to be insensitive to membrane stretch [42]. TRPC1 and TRPV2 are activated by pulsatile stretch, but there is no direct data, which could confirm their respond to shear stress [43].Generally, it has been shown that TRPs activated by stretch include TRPC1 [44] and TRPV2 [45], and on the other hand flow-activated channels are TRPV4 [46] and TRPP1/TRPP2 complex [41]. However, the sensitivity of most stretch-activated Ca2+- permeable channels to flow shear stress has not been investigated [47]. On the other hand, some findings suggest that Ca2+- permeable channels can be responsive to both membrane stretch and flow shear stress [48]. This data also has shown that stretch-activation may be the primary pathway underlying flow-induced Ca2+ sparks in endothelial cells [43].
Other studies revealed that TRPM7 channels may display mechanosensitive properties. Implication of these findings is that shear stress may induce a translocation of surface TRPM7 to the plasma membrane in vascular myocytes cells in response to high laminar flow or in areas, where endothelial cells have been damaged. TRPM7 activation results in increased influx of Ca2+ in damaged cells that subsequently may trigger cell proliferation, support formation of atherosclerotic plaques and finally induce cell death [30,49].
The mechanism of response to shear-stress was reported by Thilo et al. [50]. In the experimental shear stress, to which cultured vascular endothelial cells were exposed, three different hemodynamic conditions were applied: constant laminar flow, laminar pulsatile atheroprotective flow or laminar atheroprone bidirectional flow. It has been determined that depending on chosen mode of flow; TRPC6 and TRPV1 were significantly increased in atheroprone flow profile compared with an atheroprotective one. Similarly, atheroprone flow induced higher expression of TNF-α mRNA, what was correlated with the higher expression of TRPC6, but not TRPV1. Probably, GATA1 (globin transcription factor) and GATA4 regulate TRPC6 expression during the atheroprone shear stress [51]. Although, TRPC6 and TRPV1 channels are overexpressed in the conditions of artheroprone flow, their action on endothelium might be opposite. Activation of TRPV1 channels attenuates atherosclerosis [31], whereas TRPC6 promotes this process. Increased TRPC6 expression in branched arteries leads to vascular endothelial growth factor–mediated endothelial permeability and thrombin-induced endothelial shape change [52].
In another context, shear-stress may contribute to the cerebral arteriogenesis by activation of TRPV4 [53]. Cells of the small capillaries are anchored to the extracellular matrix and blood flow is responsible for cell deformation. Therefore, it has been suggested that shear-stress increases TRPV4 expression in the membrane and induces collateral vessel growth.
Endothelial dysfunction, inflammatory cascade and oxidative stress
Pathological changes of cerebral vessel endothelial cells may be an inciting event in the formation of cerebral aneurysms. Hemodynamic stress exerts a mechanical pressure on the surface of endothelium [30] causing diverse biochemical and physiological reactions in response. Endothelial dysfunction might be observed in certain areas of cerebral arteries, such as branch points that experience variable shear stress and flow reversal [50]. TRP channels, including TRPV4 and the TRPP1–P2 complex, are activated by shear stress [6,53,54]. The dysfunction or dysregulation of these channels may impair flowinduced vascular auto regulation. Kohler et al. have found that Ca2+ influx through endothelial TRPV4 channels triggers NO- dependent vasodilatation in endothelium and NO- and EDHF-dependent vasodilatation of small-sized vessels [54].
Malfunction of endothelial TRPP1 and TRPP2 impairs regulation of NO synthase, resulting in endothelial dysfunction, which may contribute to progress of common genetic Autosomal Dominant Polycystic Kidney Disease (ADPKD). Oancea et al. have found that TRPM7 is sensitive to flow shear stress and participates in pathological responses to arterial wall injury [30]. The exposure of vascular muscle cells to shear stress after endothelial injury leads to a significant accumulation of functional TRPM7 channels at the plasma membrane in less than 2 minutes. The rapid time course for this response suggests that TRPM7 is among the first molecules that respond to shear stress [30]. Over activation of TRPM7 after the exposure of vascular smooth muscle cells to flow shear stress facilitates the translocation of TRPM7 proteins to the plasma membrane, which results in an increase in the whole-cell TRPM7 currents. Increased TRPM7 activity may cause smooth muscle cell injury in areas in which smooth muscle cells are exposed to flow, such as in atherosclerotic regions. Under normal physiological conditions endothelial cells that are permanently exposed to higher levels of shear stress may down regulate the response to mechanical stimuli. However, under prominent hemodynamic stress or rupture of atherosclerotic lesion, vascular smooth muscle cells may respond quickly, therefore expressing higher levels of TRPM7. In response to TRPM7 activation intracellular calcium induces smooth muscle proliferation at lower levels and cell death at high levels [30].
High oxidative stress in the arterial wall initiates macrophage infiltration, humoral or cell-mediated inflammatory responses that have a significant role in aneurysm formation mostly by loss of mural cells in its wall [2]. TRPM2, TRPM4, TRPV1, TRPV2 have been functionally described on macrophages and they have a profound impact on monocyte function [3,55]. TRPV2 that is expressed on macrophages may play a critical role in early phagocytosis and points to this channel as an interesting candidate to be explored regarding a potential modulatory role of efferocytic properties of macrophages in atherogenesis [56,57].
Monocyte recruitment to the endothelium entails interaction of integrins on the monocyte surface with intercellular cell adhesion-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). TRPC3 expression is obligatory for ATP-induced VCAM-1 and ICAM-1 monocyte endothelial adhesion [58]. Both ICAM-1 and VCAM-1 have a central function not only in mediating adhesion of monocytes to the endothelial layer, but also in the endothelial intracellular signalling that supports transmigration of the bound monocyte.
TRP channels may potentially act as endothelial cell sensors for oxidative stress. TRPC3 is a component of oxidant-activated cation channels in endothelial cells. Poteser et al. have demonstrated that oxidant-activated TRPC3 cation conductance in TRPC3- overexpressing HEK cells and porcine aortic endothelial cells is inhibited by dominant negative mutants of TRPC4 [59]. TRPM2 and -M7 have an important functional role in oxidative stress-induced cell injury. The role of TRPM2 isoforms in cell proliferation and oxidant-induced cell death has been well established [60].
Structural remodelling of the arterial wall
Hemodynamic stress initiates structural remodelling of arterial wall, smooth muscle cells intensively proliferate and chaotically migrate to the intima (myointimal hyperplasia) [2]. The early stage of the wall remodelling process reflects the repairing mechanism of artery damage after the pathological hemodynamic forces had acted. An intra-luminal thrombus is being infiltrated by Smooth Muscle Cells (SMC), mural cells begin to proliferate and synthesize new collagen matrix [61]. The remodelling process, although complex in nature, is mediated by TRP channels.
TRPC6 and TRPM4, being sensors of mechanotransduction during the myogenic response induced by an increase of intraluminal pressure, are likely candidates as stretch-activated cation channels in vascular smooth muscle [27]. These channels may be directly activated by membrane stretch or by cell-signalling pathways. TRPC6 is activated by pressure-induced smooth muscle depolarization causing vasoconstriction of cerebral resistance arteries. Blocking of TRPC6 expression attenuates membrane depolarization, swellingactivated currents in cerebral myocytes and vasoconstriction induced by elevation of intraluminal pressure. TRPC6 participates in the intravascular pressure-induced depolarization and constriction of small arteries and arterioles known as the Bayliss effect [62].
TRPM4 channel contributes to stretch-induced myogenic depolarization and SMC migration after their prolongated activation in the cerebral arteries [63]. TRPC5 channel does not constrict cerebral arteries, but rather may participate in the process of SMC migration and proliferation [64].
The function of vascular myocytes is regulated by the activity of TRPC1 channel. Overexpression of this channel in cerebral arteries is associated with substantial increase in store-operated Ca2+ entry, suggesting that TRPC1 forms store-operated Ca2+ influx channels in vascular myocytes [65]. TRPC1 expression is increased during Ang II induced vascular hypertrophy and during vascular occlusive disease [66]. TRPC1 activity is also associated with SMC proliferation that occurs following activation of store-operatedCa2+ entry.
It’s presumed that TRPM7 channel mediates pathological alteration in the vascular adventitia and can be activated by stretching or shear stress in vascular SMC. The activation of TRPM7 by pressure overload-induced stretching can recruit macrophages into thickened adventitia and increase remodelling in a mechanosensitive manner. TRPM7 modulates the infiltration of macrophages and acts synergistically to promote collagen accumulation and vascular adventitial formation during pressure overload in rats with TAC (transverse aortic constriction) [55].
Non-physiological hemodynamic forces produce endothelial dysfunction resulting in collagen exposure and thrombus formation [2,67]. Probably the first degenerative change in arterial wall is damage of layer, which leads to the formation of fibrin network and thrombus on the exposed collagen surface. The abnormal Ca2+ influx in endothelial cells lacking of TRPC4 channels was associated with a lack of thrombin-mediated actin-stress fiber formation. The inability of thrombin to induce actin-stress fiber formation may be a direct result of TRPC4 interaction with protein 4.1, an endothelial cytoskeletal protein [68]. Thrombin is an inflammatory mediator that activates TRPC1. Both, the resultant Ca2+ influx and tumor necrosis factor-alpha stimulate the expression of TRPC1 in human vascular endothelial cells [69]. In response, increased expression of TRPC1 augments Ca2+ influx via store-operated channels and potentiates the thrombin-induced increase in permeability in human vascular endothelial cells [70].
The one suggestion implying the linkage between aneurysms etiopathogenesis and TRP channels derives from the study on Autosomal Dominant Polycystic Kidney Disease (ADKPD) [71,72]. ADKPD patients suffer not only from kidney cysts and arterial hypertension, but also form aneurysms as a result of thinning of the vessel wall that facilitates aneurysmal bleeding [73].The link between intracellular Ca2+ level alterations and aneurysms is not clear, but the suggestion is that TRPP channels take part in the control of the myoelastic structural integrity of arteries [74]. TRPP2 seems to be especially important for the stability of the vessel wall - the survival time of patients with TRPP2 mutations is longer than for those with TRPP1 defects [75].
TRPP1/TRPP2 complex is highly expressed in arterial smooth muscle cells and there is a likelihood, that mutations in both encoding genes have impact on structural and functional integrity of the arteries [41].The current concept is that the large terminal region of TRPP1 protein may act as a flow sensor modulating the activity of the coupled TRPP2 channel subunit, which activity remains also under the control of tubulin [76]. SAC (Stretch-Activated Channels) currents in smooth muscle cells are suppressed by TRPP2 expression, which is reversed by TRPP1 expression, indicating that TRPP1/TRPP2 expression ratio plays a regulatory role for pressure-induced Ca2+ entry SMC [74]. It is noteworthy that TRPP2 associates with TRPC1, but similarly, TRPP2 may also associate with TRPV4 channel [77]. The physiological significance of the TRPC1/ TRPP2and TRPV4/TRPP2 complexes remains to be investigated more precisely. It has recently been proposed that structural changes in microtubule-TRPP2 links may constitute a regulatory mechanism of channel function via the microtubule dependent motor kinesin-2 subunit KIF3A, also involved in the pathogenesis of ADPKD [78]. However, it is still unknown whether the loss of mechanosensation is the direct cause of ADPKD. It is possible that the TRPP complex may fulfill another function, including chemo sensation as recently demonstrated for the PKD1L3/PKD2L1 (TRPP3) complex [79].
Conclusion
In the above mention revision authors presented the role of TRP channels in the pathogenesis of cerebral aneurysms formation. Although the current results and data are based mainly on animal studies, it can be hypothesized that TRP channels allow explaining many unclear issues of cerebrovascular diseases. It seems that there should be paid more attention to TRP channels in clinical studies to confirm previous observations. Definitely, TRP channels are involved in many cerebrovascular processes that are crucial in the physiology of cerebral circulation. Their modulative abilities regulate the adequate blood flow in different hemodynamic conditions. Finally, under the pathological shear stress TRP channels transduce mechanical stimuli that initiate cascade leading to the aneurysm formation. Current knowledge justifies the conclusion that TRP channels play an important role in the process of cerebral aneurysms formation. Therefore, modulation of their activity should be taken into account in future clinical trials on cerebrovascular diseases.
References
- Shojima M, Nemoto S, Morita A, Oshima M, Watanabe E, Saito N. Role of shear stress in the blister formation of cerebral aneurysms. Neurosurgery. 2010; 67: 1268-1274.
- Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004; 35: 2287-2293.
- Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007; 38: 162-169.
- Korja M, Silventoinen K, McCarron P, Zdravkovic S, Skytthe A, Haapanen A, et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke. 2010; 41: 2458-2462.
- Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol. 2012; 2: 563-608.
- Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011; 704: 615-636.
- Koltzenburg M. The role of TRP channels in sensory neurons. Novartis Found Symp. 2004; 260: 206-213.
- Story GM. The emerging role of TRP channels in mechanisms of temperature and pain sensation. Curr Neuropharmacol. 2006; 4: 183-196.
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969; 224: 285-287.
- Abriel H, Syam N, Sottas V, Amarouch MY, Rougier JS. TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology. Biochem Pharmacol. 2012; 84: 873-881.
- Earley S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation. 2010; 17: 237-249.
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002; 9: 229-231.
- Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 2008; 29: 1761-1767.
- Malek AM, Izumo S. Molecular aspects of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994; 12: 989-999.
- Jose AM, Bany IA, Chase DL, Koelle MR. A specific subset of transient receptor potential vanilloid-type channel subunits in Caenorhabditis elegans endocrine cells function as mixed heteromers to promote neurotransmitter release. Genetics. 2007; 175: 93-105.
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010; 67: 381-391.
- Köhler R, Hoyer J. Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells. Liedtke WB, Heller S, editors. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades [Internet]. Boca Raton (FL): CRC Press; 2007.
- Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond). 2010; 119: 19-36.
- Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol. 2011; 57: 133-139.
- Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009; 123: 371-385.
- Antoniotti S, Fiorio Pla A, Barral S, Scalabrino O, Munaron L, Lovisolo D. Interaction between TRPC channel subunits in endothelial cells. J Recept Signal Transduct Res. 2006; 26: 225-240.
- Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013; 61: 113-119.
- Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, et al. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009; 104: 670-678.
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008; 27: 3092-3103.
- Chakraborty S, Berwick ZC, Bartlett PJ, Kumar S, Thomas AP, Sturek M, et al. Bromoenol lactone inhibits voltage-gated Ca2+ and transient receptor potential canonical channels. J Pharmacol Exp Ther. 2011; 339: 329-340.
- Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. J Physiol. 2011; 589: 1527-1534.
- Kim EC, Choi SK, Lim M, Yeon SI, Lee YH. Role of endogenous ENaC and TRP channels in the myogenic response of rat posterior cerebral arteries. PLoS One. 2013; 8: e84194.
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007; 8: 510-521.
- Marrelli SP, O'neil RG, Brown RC, Bryan RM Jr. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007; 292: H1390-1397.
- Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006; 98: 245-253.
- Ma L, Zhong J, Zhao Z, Luo Z, Ma S, Sun J, et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. 2011; 92: 504-513.
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010; 298: H466-476.
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, et al. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One. 2007; 2: e827.
- Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge Z-D, Li R, et al. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009; 53: 532-538.
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004; 101: 396-401.
- Wegierski T, Lewandrowski U, Müller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009; 284: 2923-2933.
- Kotlikoff MI. EDHF redux: EETs, TRPV4, and Ca2+ sparks. Circ Res. 2005; 97: 1209-1210.
- Fernández-Fernández JM, Andrade YN, Arniges M, Fernandes J, Plata C, Rubio-Moscardo F, et al. Functional coupling of TRPV4 cationic channel and large conductance, calcium-dependent potassium channel in human bronchial epithelial cell lines. Pflüg Arch Eur J Physiol. 2008; 457: 149-159.
- Chen XZ, Li Q, Wu Y, Liang G, Lara CJ, Cantiello HF. Submembraneous microtubule cytoskeleton: interaction of TRPP2 with the cell cytoskeleton. FEBS J. 2008; 275: 4675-4683.
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010; 5: e12177.
- Narayanan D, Bulley S, Leo MD, Burris SK, Gabrick KS, Boop FA, et al. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J Physiol. 2013; 591: 5031-5046.
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000; 2: 695-702.
- Rowell J, Koitabashi N, Kass DA. TRP-ing up heart and vessels: canonical transient receptor potential channels and cardiovascular disease. J Cardiovasc Transl Res. 2010; 3: 516-524.
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005; 7: 179-185.
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003; 93: 829-838.
- Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003; 278: 27129-27137.
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005; 97: 853-863.
- Yao X, Kwan HY, Chan FL, Chan NW, Huang Y. A protein kinase G-sensitive channel mediates flow-induced Ca(2+) entry into vascular endothelial cells. FASEB J. 2000; 14: 932-938.
- Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007; 19: 1-8.
- Thilo F, Vorderwülbecke BJ, Marki A, Krueger K, Liu Y, Baumunk D, et al. Pulsatile atheroprone shear stress affects the expression of transient receptor potential channels in human endothelial cells. Hypertension. 2012; 59: 1232-1240.,
- Fan C, Ouyang P, Timur AA, He P, You SA, Hu Y, et al. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009; 284: 23331-23343.
- Pocock TM, Foster RR, Bates DO. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol. 2004; 286: H1015-1026.
- Schierling W, Troidl K, Apfelbeck H, Troidl C, Kasprzak PM, Schaper W, et al. Cerebral arteriogenesis is enhanced by pharmacological as well as fluid-shear-stress activation of the Trpv4 calcium channel. Eur J Vasc Endovasc Surg. 2011; 41: 589-596.
- Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012; 49: 375-389.
- Li Y, Jiang H, Ruan C, Zhong J, Gao P, Zhu D, et al. The interaction of transient receptor potential melastatin 7 with macrophages promotes vascular adventitial remodeling in transverse aortic constriction rats. Hypertens Res Off J Jpn Soc Hypertens. 2014; 37: 35-42.
- Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010; 11: 232-239.
- Nagasawa M, Kojima I. Translocation of calcium-permeable TRPV2 channel to the podosome: Its role in the regulation of podosome assembly. Cell Calcium. 2012; 51: 186-193.
- Smedlund K, Tano JY, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010; 106: 1479-1488.
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, et al. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 2006; 281: 13588-13595.
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002; 9: 163-173.
- Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000; 190: 300-309.
- Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002; 90: 248-250.
- Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke. 2007; 38: 2322-2328.
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006; 281: 4977-4982.
- Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, et al. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005; 288: C872-880.
- Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, et al. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res. 2008; 103: e97-104.
- Lavallée PC, Labreuche J, Faille D, Huisse M-G, Nicaise-Roland P, Dehoux M, et al. Circulating Markers of Endothelial Dysfunction and Platelet Activation in Patients with Severe Symptomatic Cerebral Small Vessel Disease. Cerebrovasc Dis [Internet]. 2013; 36: 131-138.
- Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005; 97: 1164-1172.
- Paria BC, Bair AM, Xue J, Yu Y, Malik AB, Tiruppathi C. Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-kappaB activation in endothelial cells. J Biol Chem. 2006; 281: 20715-20727.
- Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006; 13: 693-708.
- Qamar S, Vadivelu M, Sandford R. TRP channels and kidney disease: lessons from polycystic kidney disease. Biochem Soc Trans. 2007; 35: 124-128.
- Roth C, Kleffmann J, Bergmann C, Deinsberger W, Ferbert A. Ruptured cerebral aneurysm and acute bilateral carotid artery dissection in a patient with polycystic kidney disease and polycystic liver disease. Cerebrovasc Dis Basel Switz. 2013; 35: 590-591.
- Neumann HP, Malinoc A, Bacher J, Nabulsi Z, Ivanovas V, Bruechle NO, et al. Characteristics of intracranial aneurysms in the else kröner-fresenius registry of autosomal dominant polycystic kidney disease. Cerebrovasc Dis Extra. 2012; 2: 71-79.
- Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006; 112: 744-760.
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007; 87: 165-217.
- Sharif-Naeini R, Dedman A, Folgering JH, Duprat F, Patel A, Nilius B, et al. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflugers Arch. 2008; 456: 529-540.
- Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, et al. TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One. 2013; 8: e73424.
- Boehlke C, Kotsis F, Buchholz B, Powelske C, Eckardt KU, Walz G, et al. Kif3a guides microtubular dynamics, migration and lumen formation of MDCK cells. PLoS One. 2013; 8: e62165.
- Yu Y, Ulbrich MH, Li MH, Dobbins S, Zhang WK, Tong L, et al. Molecular mechanism of the assembly of an acid-sensing receptor ion channel complex. Nat Commun. 2012; 3: 1252.