
Case Report
Austin Neurosurg Open Access. 2015; 2(3): 1033.
Melanotic Schwannoma of the Jugular Foramen with Rapid Necrotic Regression Mimicking an Intratumoral Hemorrhage of the Jugular Foramen Neurinoma
Yu Teranishi¹*, Michihiro Kohno¹, Shigeo Sora¹, Hiroaki Sato¹, Munehiro Yokoyama² and Yohichi Nakazato³
1Department of Neurosurgery, Tokyo Metropolitan Police Hospital, Japan
2Department of Pathology, Tokyo Metropolitan Police Hospital, Japan
3Department of Pathology, Hidaka Hospital, Japan
*Corresponding author: Yu Teranishi, Department of Neurosurgery, Tokyo Metropolitan Police Hospital, 4-22- 1 Nakano, Nakano-ku, Tokyo, Japan
Received: July 11, 2015; Accepted: July 27, 2015; Published: July 29, 2015
Abstract
Melanotic tumors of the Cerebellopontine Angle (CPA) are rare. On the other hand, intratumoral hemorrhages of the intracranial schwannoma are occasionally seen. It is difficult to preoperatively differentiate these tumors by Magnetic Resonance Imaging (MRI), especially in a case with acute onset symptoms. The authors describe a unique case of a jugular foramen melanotic tumor with acute onset symptoms. A 32-year-old woman presented with sudden onset dysphagia. MRI revealed a 2-cm extra axial tumor around the jugular foramen. The tumor showed hyperintensity on T1-weighted images and hypointensity on T2-weighted images, which showed a lack of enhancement with intravenous administration of gadolinium. Based on the sudden onset of symptoms and MRI findings, we diagnosed the tumor as a jugular foramen schwannoma with intratumoral hemorrhage. At surgery, the tumor was found to originate from the XIth cranial nerve by direct visualization under the surgical microscope, but the tumor was composed of a black-colored tissue that was obviously different from an intratumoral hemorrhage. Pathologically, the tumor content was full of melanophage and necrotic tissue, but tumor cells were not identified. The tumor capsule includes S-100 positive fibers. Based on these findings, our diagnosis was a melanotic schwannoma of the XIth cranial nerve with rapid necrotic regression. Even though the CPA tumor showed hyperintensity on T1-weighted images with acute onset symptoms, it is important to consider a melanotic tumor as an alternative in the differential diagnosis.
Keywords: Melanotic schwannoma; Intratumoral hemorrhages; Jugular foramen neurinoma
Introduction
Melanotic tumors of the Cerebellopontine Angle (CPA) that show hyperintensity on T1-weighted images are rare [1-4]. Differential diagnoses of a CPA melanotic tumor include metastatic malignant melanoma, melanotic schwannoma, and meningeal melanocytoma [5,6]. Except for the Carney complex and a history of malignant melanoma, there are no typical symptoms. For a melanotic tumor of the CPA, hyperintensity of T1-weighted images is a characteristic radiological finding that distinguishes it from ordinal CPA tumors. On the other hand, intratumoral hemorrhage is occasionally observed in CPA schwannomas [1,4,7,8]. Because subacute phages of intratumoral hemorrhages may show hyperintensity on T1-weighted images, a melanotic tumor of the CPA tends to be confused with intratumoral hemorrhage of a CPA tumor. In this report, we present a unique case of jugular foramen melanotic schwannoma with acute onset symptoms.
Case Report
History and examination
A 32-year-old woman suddenly presented to the local hospital with hoarseness, dysphagia. There, she was diagnosed with influenza and her symptoms worsened during one month. She was referred to our hospital for further examination and treatment. On admission, she presented with hoarseness, dysphagia, weight loss, and neck pain. Cranial nerve examination revealed failure of the left soft plate elevation and immobilization of the left vocal cord, which was fixed at midline. Dysgeusia and sternocleidomastoid muscle atrophy were also found. She had not experienced hearing loss, facial paresis, or tongue deviation. Magnetic Resonance Imaging (MRI) showed a 2-cm extra axial tumor located in the left jugular foramen. This extra axial tumor revealed heterogeneous hyperintensity on T1-weighted images, hypointensity on T2-weighted images, and hypointensity on Diffusion-Weighted Images (DWI), the latter of which did not show significant enhancement with intravenous administration of gadolinium (Figure 1). Angiography showed no tumor stain and no abnormal vessels. Based on the radiographic images, the sudden onset of symptoms, and the prevalence, intratumoral hemorrhage of a jugular foramen neurinoma was suspected. Thus, we decided to perform a surgical resection.
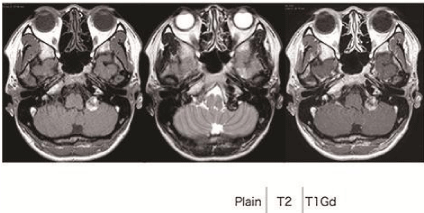
Figure 1: On preoperative MRI findings, the left CPA tumor was a 2-cm
extra axial mass and located in the left jugular foramen. This extra axial
mass revealed heterogeneous hyperintensity on T1WI and hypointensity on
T2WI, which showed a lack of enhancement with intravenous administration
of gadolinium.
Surgical intervention
Based on the radiographic images, we evaluated that it was possible to perform gross total resection of the tumor via the lateral suboccipital approach through opening the jugular foramen by drilling (Figure 2) [9].
After left suboccipital craniotomy, we retracted the cerebellum gradually, and opened the cerebello-medullary cistern. Surrounding the jugular foramen, the tumor was exposed (Figure 2A). The surface of the tumor was relatively smooth, and the tumor capsule was found. Under direct visualization via surgical microscope, we identified that the tumor originated at the spinal root of the XIth cranial nerve (Figure 2B). The Xth cranial nerve runs along the dorsal surface of the tumor, but there was no response by electric stimulation (Figure 2C). The VIIth and VIIIth cranial nerves were located ventro-rostrally to the tumor (Figure 2D). The tumor contained black necrotic tissue that was obviously different from an intratumoral hemorrhage (Figure 2E). The intraoperative pathological report revealed that the tumor content was abundant in melanin and necrotic tissue. We drilled the dorsal part of the jugular foramen, and gross total resection was achieved (Figure 3B). Postoperative MRI revealed that total resection of the tumor had been successful (Figure 3A).
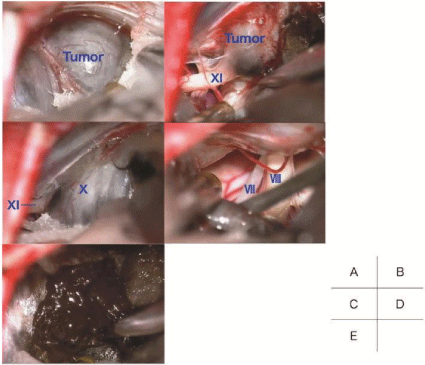
Figure 2: Intraoperative photos.
A: After we retracted the cerebellum, the tumor was exposed around the
jugular foramen. B: Under direct visualization of a surgical microscope, we
identified that the tumor originated in the spinal root of the XIth cranial nerve.
C: The Xth cranial nerve runs along the dorsal surface of the tumor, but there
was no response by electric stimulation. D: The VIIth and VIIIth cranial nerves
were located ventro-rostrally to the tumor. E: The tumor contained black
necrotic tissue that was obviously different from an intratumoral hemorrhage.
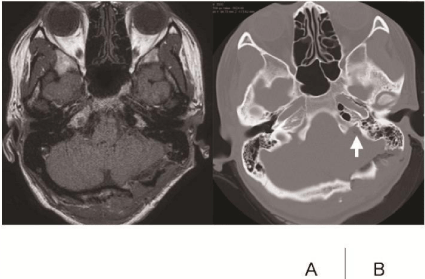
Figure 3: Postoperative radiological findings.
A: T1-Gd showed gross total resection. B: Bone window CT showed that the
dorsal part of the jugular foramen was drilled out (white arrow).
Histopathological findings
The tumor tissue was stained with H&E and other immunohistochemical stains. It had a tumor capsule containing collagen fibers (Figure 4A) and was composed of necrotic tissue, most of which was melanin and melanophage (Figure 4B and 4C). On the sections stained for S-100, the tumor capsule consisted of myelinated nerve fibers (Figure 4D). However, the tumor was filled with necrotic tissue and no tumor cell was observed. The tumor contents were positive for melanin with Masson-Zimmermann stain (Figure 4E). Based on the above histological findings, this tumor was classified as a melanotic tumor.
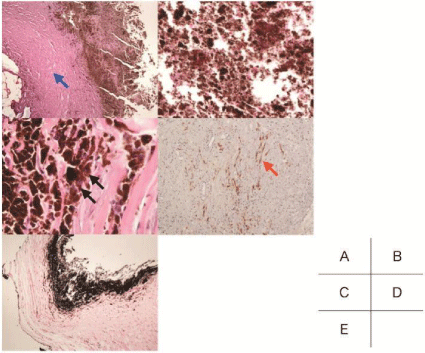
Figure 4: Pathological findings.
A: The tumor has a tumor capsule which contains collagen fibers (blue
arrow; H&E, original magnification×20). B: Tumor contents are composed of
necrotic tissue, and lack tumor cells (H&E, original magnification×20). C: In
the necrotic tissue, melanophages were identified (black arrow; H&E, original
magnification×400). D: The tumor capsule consisted of medullated nerve
fibers that were positive for s-100 (red arrow). E: Tumor contents are positive
for melanin with Masson-Zimmermann stain.
Postoperative course
The patient’s postoperative course was uneventful. Her dysphagia and hoarseness worsened temporarily, but most symptoms were resolved within six months. At one year after the operation, MRI showed no recurrence of the tumor.
Discussion
Although no tumor cell was observed in the tumor content, we diagnosed this melanotic tumor as a melanotic schwannoma, based on intraoperative findings and pathological findings. In surgery, the tumor was determined to have originated at the spinal root of the XIth cranial nerve under direct visualization of a surgical microscope (Figure 2B). The tumor capsule was relatively thick and smooth. There was no continuity with the dura matter. Moreover, on the sections stained for S-100, myelinated nerve fibers were identified in the tumor capsule (Figure 4D). This pathological finding is often observed in neurofibroma, and it explains that this tumor could have originated in the peripheral nerve.
The reason why no tumor cells were observed is unclear. Necrotic change was known as a characteristic finding in malignant tumors, and was thought to be induced by microcirculation impairment with acute tumor growth. However, the necrotic tissue in this case was not surrounded by malignant tumor cells. Thus, this necrotic change was not relevant to that of malignant findings. In terms of intracranial schwannoma, this necrotic change is observed occasionally after gamma knife therapy. A tumor that receives gamma knife therapy experiences necrotic change, pseudo enlargement by swelling, and tumor death [10]. However, our patient did not receive any radiation therapy.
Infarcted schwannoma has previously been reported [11,12]. In these reports, an acute infarcted tumor is caused by arterial thrombosis or marked venous congestion, and the infarcted condition may cause acute compression of surrounding nerves by swelling of the lesion. Characteristically, an acute infarcted tumor shows a lack of enhancement, and is accompanied by acute onset of symptoms. In our case, the patient suddenly presented with lower cranial nerve symptoms, and the tumor was filled with necrotic tissue. Above all, we predicted that focal microcirculation impairment induced rapid necrotic change, i.e., tumor “apoplexy”. And this caused her lower cranial nerve symptoms by compression. Thus, we diagnosed this as melanotic schwannoma of the jugular foramen with rapid necrotic regression.
Melanotic schwannoma is a rare tumor, most commonly occurring in the paraspinal lesion, and is associated with the Carney complex [6]. It has been reported that the tumor origin of a melanotic schwannoma is found in the Vth, VIIth and VIIIth cranial nerves [13-16]. To our knowledge, however, a melanotic schwannoma originating at the XIth cranial nerve has not previously been reported.
Because the biological behavior, treatment and prognosis of each diagnosis varies, it is important to preoperatively distinguish. In terms of MRI findings, the melanotic tumor showed hyperintensity on T1- weighted images and hypointensity on T2-weighted images [1,3,4]. These findings could be diagnostic, but we need to carefully consider that intratumoral hemorrhage produces similar MRI findings.
Malignant transformation of each melanotic tumor has been previously reported. Malignant varieties of the melanotic schwannoma are known to comprise about 10% of all cases [6]. In this case, it was difficult to predict the tumor malignancy because the tumor was filled with necrotic tissue and no tumor cell was observed. Actually, we discussed whether the lower cranial nerve should be sacrificed or not during the surgery. Sacrificing the lower cranial nerve comes with the risk of worsening her symptoms severely, which would require tracheostomy and long-term rehabilitation. But it may also decrease the risk of a recurrence. On the other hand, in the worst-case scenario, preserving the contents of the jugular foramen may cause a recurrence, resulting in the need for further invasive procedures. Because her vocal cord was already fixed at the midline, we expected that her symptoms might not be worsened severely, even if we sacrificed the lower cranial nerve. Taking those conditions into consideration, we decided to resect all of the contents of the jugular foramen.
References
- Jaiswal S, Vij M, Tungria A, Jaiswal AK, Srivastava AK, Behari S. Primary melanocytic tumors of the central nervous system: a neuroradiological and clinicopathological study of five cases and brief review of literature. Neurol India. 2011; 59: 413-419.
- Sengöz A, Taşdemiroğlu E, Togay H. Is clear cell sarcoma a malignant form of psammomatous melanotic schwannoma? Case report. Neurosurg Focus. 2006; 21: E11.
- Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: radiologic-pathologic correlation. Radiographics. 2009; 29: 1503-1524.
- Uematsu Y, Yukawa S, Yokote H, Itakura T, Hayashi S, Komai N. Meningeal melanocytoma: magnetic resonance imaging characteristics and pathological features. Case report. J Neurosurg. 1992; 76: 705-709.
- Gupta A, Ahmad FU, Sharma MC, Garg A, Mehta VS. Cerebellopontine angle meningeal melanocytoma: a rare tumor in an uncommon location. Case report. J Neurosurg. 2007; 106: 1094-1097.
- Piedra MP, Scheithauer BW, Driscoll CL, Link MJ. Primary melanocytic tumor of the cerebellopontine angle mimicking a vestibular schwannoma: case report. Neurosurgery. 2006; 59: E206.
- Guinto Balanzar G, Guinto-Nishimura Y. Intratumoral hemorrhage in vestibular schwannomas. World Neurosurg. 2014; 82: 599-600.
- Niknafs YS, Wang AC, Than KD, Etame AB, Thompson BG, Sullivan SE. Hemorrhagic vestibular schwannoma: review of the literature. World Neurosurg. 2014; 82: 751-756.
- Matsushima K, Kohno M, Komune N, Miki K, Matsushima T, Rhoton AL Jr. Suprajugular extension of the retrosigmoid approach: microsurgical anatomy. J Neurosurg. 2014; 121: 397-407.
- Hasegawa T, Kida Y, Yoshimoto M, Koike J, Goto K. Evaluation of tumor expansion after stereotactic radiosurgery in patients harboring vestibular schwannomas. Neurosurgery. 2006; 58: 1119-1128.
- Khoshyomn S, Barth KN, Christman RA, Braff SP, Wilson JT. Torsion of a lumbar nerve root schwannoma. Pediatr Neurosurg. 2002; 37: 206-209.
- Shrier DA, Rubio A, Numaguchi Y, Powers JM. Infarcted spinal schwannoma: an unusual MR finding. AJNR Am J Neuroradiol. 1996; 17: 1566-1568.
- Dastur DK, Sinh G, Pandya SK. Melanotic tumor of the acoustic nerve: case report. 1966.
- Earls JP, Robles HA, McAdams HP, Rao KC. General case of the day. Malignant melanotic schwannoma of the eighth cranial nerve. Radiographics. 1994; 14: 1425-1427.
- Killeen RM, Davy CL, Bauserman SC. Melanocytic schwannoma. Cancer. 1988; 62: 174-183.
- Miller RT, Sarikaya H, Sos A. Melanotic schwannoma of the acoustic nerve. Arch Pathol Lab Med. 1986; 110: 153-154.