
Research Article
Austin Neurosurg Open Access. 2015; 2(3): 1036.
Gingko Biloba versus Neuropeptide Derivative FPF 1070 (Cerebrolysin) Effect against Cisplatin-Induced Sciatic Neuropathy
Ereny FY Makary, Eman AI Ali*, Lamia Fargali and Somaya Hosny
Department of Histology, Suez Canal University, Egypt
*Corresponding author: Ali EAI, Department of Histology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Received: June 22, 2015; Accepted: September 07, 2015; Published: September 09, 2015
Abstract
Introduction: Cisplatin is one of the most widely prescribed antineoplastic agents. It has shown to be effective against various malignant neoplasms. However, its clinical use is limited due to its various side effects including peripheral neuropathy. Cerebrolysin; a neuroprotective agent, was effectively used against diabetic neuropathy and other neurodegenerative diseases. Meanwhile, Gingko biloba showed to be effective against various central nervous system disorders such as dementia, Alzheimer’s disease and cerebral insufficiency.
Aim: To study the protective effects of Gingko biloba versus cerebrolysin against cisplatin-induced sciatic neuropathy in adult male albino rat.
Materials and Methods: Animals were randomized into 6 groups, 10 rats in each group, Group 1 (control), Group 2 (received daily I.P. injection of2.5 ml/ kg of cerebrolysin for 4 weeks), Group 3 (received100 mg/kg of Gingko biloba orally daily for 4 weeks using oral gavage), Group 4 (received I.P. injection of 2 mg/kg cisplatin twice a week for 4 weeks), Group 5 (combined treatment of cerebrolysin and cisplatin for 4 weeks) and Group 6 (combined treatment of Gingko biloba and Cisplatin for 4 weeks).
Results: Cisplatin caused marked disorganization and/or shrinkage of myelinated nerve fibers with severe compression of the axoplasm. Cerebrolysin exerted more protective effect against Cisplatin induced neuropathy than that of Gingko biloba.
Conclusion: Cerebrolysin and Gingko biloba exerted a neuroprotective effect against cisplatin-induced peripheral neuropathy, but cerebrolysin showed more improvement.
Keywords: Cisplatin; Sciatic neuropathy; Cerebrolysin; Gingko biloba
Abbreviations
BW: Body weight; DRG: Dorsal Root Ganglia; EGb761: Gingko Biloba Extract 761; FPF1070: Fine Particle Fraction 1070; GSH: Glutathione; H&E: Hematoxyline and Eosin; I.P.: Intraperitoneal; LM: Light Microscope; ROS: Reactive Oxygen Species; SEM: Standard Error of the Mean; SPSS: Statistical Package of Social Sciences; TEM: Transmission Electron Microscope
Introduction
Cisplatin (Cis-diamminedichloroplatinum II) is the first member of the platinum coordination complex class of the anticancer drugs [1]. It has shown to be effective against a broad range of various solid tumors including cancers of the breast, brain, head and neck, stomach, lung, bladder, testis and ovary [2]. However; its clinical use is limited due to its severe side effects including nephrotoxicity, hepatotoxicity, ototoxicity, myelosupression, gastrointestinal and retinal toxicity in addition to peripheral neuropathy [3-5].
Cisplatin-induced peripheral neuropathy represents a major clinical problem. It is estimated that 10-40% of patients treated with cisplatin suffer from severe peripheral neuropathy [6,7]. The clinical features of this neuropathy are mainly sensory including paraesthesia, allodynia or burning pain [8,9]. Cisplatin neurotoxicity was explained by several mechanisms such as its ability to accumulate in the Dorsal Root Ganglia (DRG) leading to degeneration of their large sensory neurons, disruption of microtubule dynamics, apoptosis and vascular neurotoxicity which is triggered mainly by oxidative stress caused by increased Reactive Oxygen Species (ROS) [10-13].
Thus, it is crucial to find neuroprotective agents against cisplatininduced peripheral neuropathy, not interfering with its antineoplastic activity. A number of neuroprotective agents have shown promising results such as chelating, antioxidants & neurotrophic factors [12,14- 16]. Cerebrolysin, a neuropeptide derivative FPF 1070, is a synthetic preparation that mimics the action of endogenous neurotrophic factors. Such factors are used as a part of therapeutic strategy that can repair and protect the brain from pathological damage of acute and chronic neurological disorders including dementia, Alzheimer’s disease, stroke and traumatic brain injury. It also showed beneficial effects against peripheral diabetic neuropathy in type 2 diabetes mellitus [17-19]. Meanwhile; Gingko biloba extract EGb761, a potent natural compound extracted from Gingko biloba leaves, improved mental and cognitive functions in various central nervous system disorders such as dementia and cerebral insufficiency. It also showed a protective effect in ischemia/ reperfusion brain injuries and spinal cord injury in animal models [20-23].
Thus; the present study was designed to investigate the possible neuroprotective effect of ginko biloba versus cerebrolysin against cisplatin-induced sciatic neuropathy.
Materials and Methods
Chemicals
Cerebrolysin used in this study was produced by Ebewe Pharmaceuticals in the form of ampoules containing 1ml or 5ml cerebrolysin. Gingko biloba was produced by Ema Pharm Company in the form of capsules, each containing 260 mg. The capsule was dissolved in 52 ml distilled water. Accordingly, the dose of a rat (150 g) is 15 mg dissolved in 3 ml distilled water. Cisplatin (Cisplatyl 50) was produced by Laboratoire Roger Bellon, France.
Experimental animals
Sixty adult male albino rats of the same age & weight (130- 150 g) were used in this study. The animals were acclimatized in suitable cages for 1 week before the experiment, housed under standardized conditions; with free access to standard pellet animal diet and tap water. Animals were anaesthetized before scarification. The experiment was performed in the Histology department, Faculty of Medicine, Suez Canal University. Approval of the research committee of the school was taken for ethical consideration of dealing with animals.
Experimental design
The animals were randomized into 6 groups (n=10 rats) (i) Control group (G1): received daily Intraperitoneal (I.P) injection of 1 ml 0.9 % saline for 4weeks; (ii) Cerebrolysin group (G2): received daily I.P. injection of 2.5 ml/ kg Body Weight (BW) of Cerebrolysin for 4 weeks; (iii) Gingko biloba group (G3): received 100 mg/kg BW Gingko biloba orally by oral gavage, daily for 4 weeks; (iv) Cisplatin group (G4):received I.P injection of 2 mg/kg BW Cisplatin, twice a week for 4 weeks; (v) Cerebrolysin + Cisplatin group (G5): received daily I.P. injection of 2.5 ml/ kg BW Cerebrolysin combined with I.P injection of 2 mg/kg BW Cisplatin twice a week, for 4 weeks; (vi) Gingko biloba + Cisplatin group (G6): received 100 mg/kg BW Gingko biloba orally combined with I.P injection of 2 mg/kg BW Cisplatin twice a week, for 4 weeks. At the end of the experiment; all animals were anesthetized using I.P. injection of 90 mg/kg BW ketamine and then sacrificed. The sciatic nerve was exposed through an incision along the veins which run between the semi-tendinosus and the biceps muscle. Then, it was excised and processed for light and transmission electron microscopic examination.
Processing for Light Microscopic (LM) examination
The sciatic nerve was dissected and cut into small segments and H&E stained paraffin sections, 5 μm thick, were prepared. Qualitative assessment was performed by the examination of 5 high power fields (X400) in 10 serial sections from each animal of all the studied groups, to assess the integrity of axons (preserved/lost) and the space of myelin (Well-organized/Disorganized/obliterated).
Processing for Transmission Electron Microscopic (TEM) examination
A very small piece (1-2 mm in length) of the sciatic nerve was cut and processed as described by Bozzola & Russell [24]. Nerve samples were fixed in a mixture of 2.5% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M Cacodylate buffer for 24 hours at 4 CO, post fixed for 2 hours in 1% buffered OSO4 and dehydrated by ascending grades of alcohol and cleared in propylene oxide. Then, nerve samples were embedded in absolute resin, sectioned transversely into Semithin (0.5 μm) sections which were stained with toluidine blue and examined by light microscope. Ultrathin (40- 50nm) sections, cut with ultra microtome and stained with 4% uranyl acetate and 2% lead citrate, were examined using Transmission Electron microscope (in Transmission Electron Microscope Unit, Ain Shams Specialized Hospital & Alazhar University) to detect histopathological changes in the axon, myelin sheath, Schwann cells and unmyelinated nerve fibers.
Statistical Analysis
The frequency distribution of each change was calculated, Data were analyzed using the Statistical Package of Social Sciences (SPSS) program version 17, (Chicago, IL, USA). Comparisons among groups were carried out using one-way ANOVA. Histopathological scoring, statistical analysis was performed using nonparametric tests. All p values reported are two-tailed and p < 0.05 was considered significant.
Results
Clinical observations
No mortality was recorded among animals of different groups. However, Five to six days after cisplatin treatment, marked weight loss was observed in animals of Cisplatin group compared to other groups.
Microscopic examination
(i) Control group (G1)
In the H&E stained sections, all animals showed normal appearance of sciatic nerve. Each nerve bundle was surrounded by connective tissue perineurium. It contained large and small myelinated nerve fibers separated by connective tissue endoneurium. Each nerve fiber has a central axon, surrounded by space of dissolved myelin. Crescent- shaped nuclei of Schwann cells, some nuclei of endoneurial cells and blood vessel were also seen (Table 1) (Figure 1).
Group Histopathological changes
Control
(G1)
Cisplatin
(G4)
Cisplatin
+
Cerebrolysin
(G5)
Cisplatin
+
Gingko biloba
(G6)
Well organized myelin space
100
0a
82b
55b
Obliteration of myelin spaces
0
100a
18b
45a,b
Preserved axons
100
10a
100b
63b
Loss of axons
0
90a
0b
37b
Values are of percentages (%) (n = 10 rats/group).
a p < 0.0001compared to G1.
b p < 0.0001compared to G4.
Table 1: The frequency distribution of the different histopathological changes of sciatic nerves in different groups.
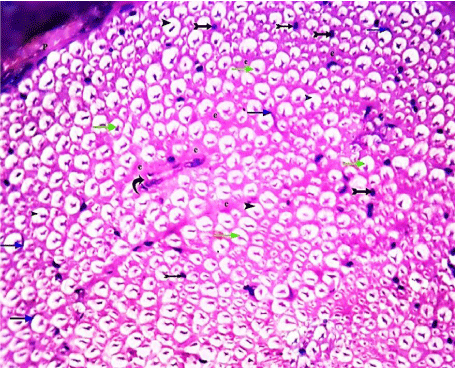
Figure 1: A transverse section of sciatic nerve from the control group, showing
part of a nerve bundle surrounded by connective tissue Perineurium (P). It
contains large and small myelinated nerve fibers separated by connective
tissue Endoneurium (e). Each nerve fiber has a central axon (green arrows),
surrounded by space of myelin (arrow heads). Crescent- shaped nuclei of
Schwann cells (blue arrows), some nuclei of endoneurial cells (tailed arrows)
and blood vessels (curved arrow) are occasionally shown. (H&E x 400).
Toluidine blue semithin sections also showed part of nerve bundle in which the myelinated nerve fibers have compact regular myelin sheaths that have darkly stained rounded or elliptical shapes. The unmyelinated nerve fibers and Schwann cells were also occasionally seen (Figure 2).
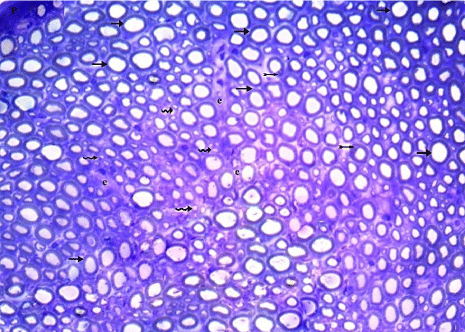
Figure 2: Myelinated nerve fibers (arrows) have compact regular myelin
sheaths that have darkly stained rounded or elliptical shapes. Unmyelinated
nerve fibers (wavy arrows) and Schwann cells (tailed arrows) are occasionally
shown. (Toluidine blue x 400).
Transmission Electron Microscope (TEM): Myelinated nerve fibers of different sizes were surrounded by compact regular myelin sheaths with narrow Schmidt-Lanterman clefts. Schwann cells enclosed euchromatic nuclei. Clusters of unmyelinated nerve fibers, containing several rounded or ovoid axons, embedded in Schwann cells processes were also seen. The myelinated nerve fibers showed homogenous axoplasm with intact mitochondria and regular cristae (Figures 3,4 and 5).
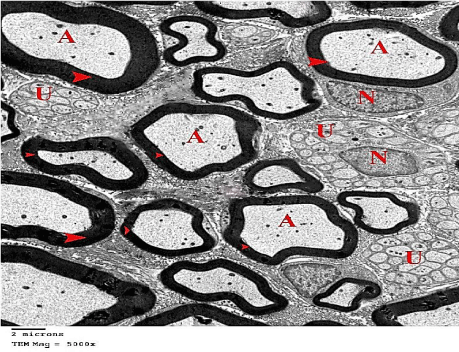
Figure 3: An electron micrograph of sciatic nerve section from the control
group, showing myelinated nerve fibers of different sizes with compact regular
myelin sheaths (arrow heads) surrounding their Axoplasm (A). Schwann cells,
surrounding myelinated nerve fiber, with its Euchromatic Nuclei (N) are also
shown. Groups of Unmyelinated Nerve Fibers (U) embedded in Schwann
cells processes, are also shown. (TEM x 5000).

Figure 4: Myelinated nerve fibers contain homogenous Axoplasm (A), with
narrow Schmidt-Lanterman Clefts (C). E= Endoneurium between the nerve
fibers. (TEM x 15000).
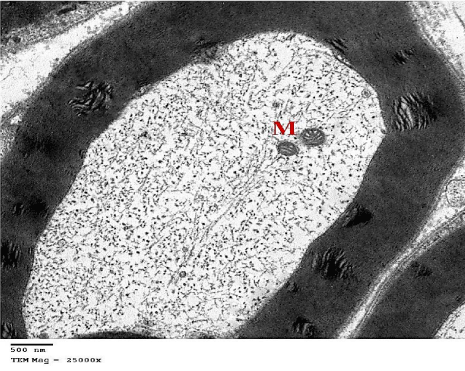
Figure 5: An electron micrograph of sciatic nerve section from the control
group, showing myelinated nerve fiber with its axoplasm containing intact
Mitochondria (M) with regular cristae. (TEM x 25000).
(ii) Cerebrolysin group (G2)
H&E stained sections, Toluidine blue semithin sections and ultrathin sections of all animals were nearly similar to control group showing normal structure of sciatic nerve (Figures 6,7 and 8).
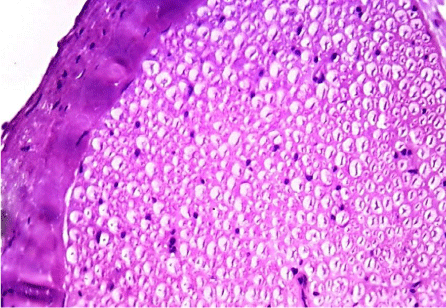
Figure 6: A transverse section of sciatic nerve from G2, showing normal
structure of sciatic nerve. (H&E x 400).
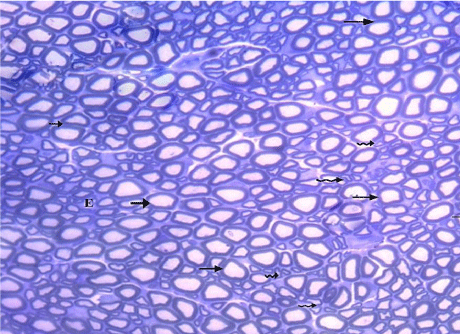
Figure 7: A semithin section of the sciatic nerve from G2, nearly similar to
control group showing myelinated (arrows) and unmyelinated (wavy arrows)
nerve fibers. (Toluidine blue x 400).
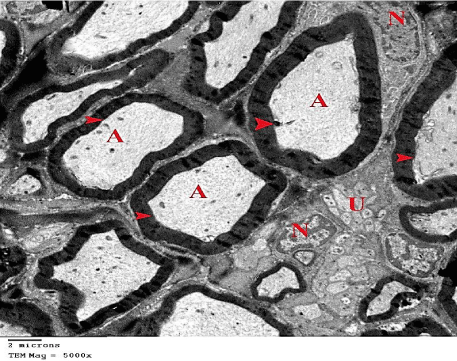
Figure 8: An electron micrograph of sciatic nerve section from G2showing
myelinated nerve fibers (arrow heads), their Axoplasm (A), Unmyelinated
nerve fibers (U) and Euchromatic Nuclei of the Schwann cells (N). (TEM x
5000).
(iii) Gingko biloba group (G3)
H&E stained sections, Toluidine blue semithin sections and ultrathin sections also showed normal structure of the sciatic nerve (Figures 9,10 and 11).
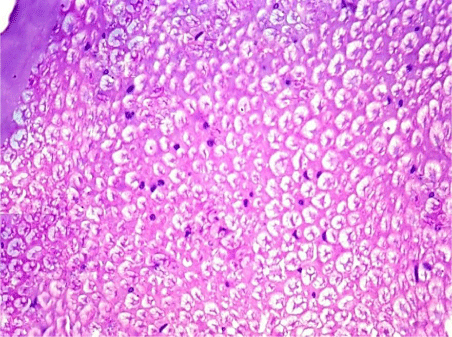
Figure 9: A transverse section of sciatic nerve from G3, more or less similar
to G1 & G2. (H&E x 400).
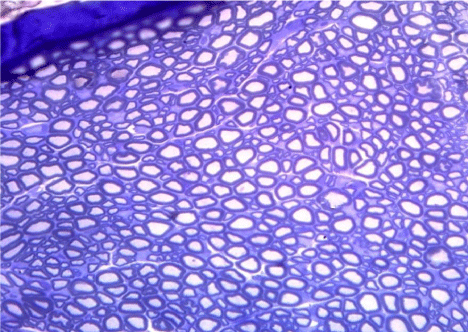
Figure 10: A semithin section of the sciatic nerve from G3, nearly similar to
G1 & G2. (Toluidine blue x 400).
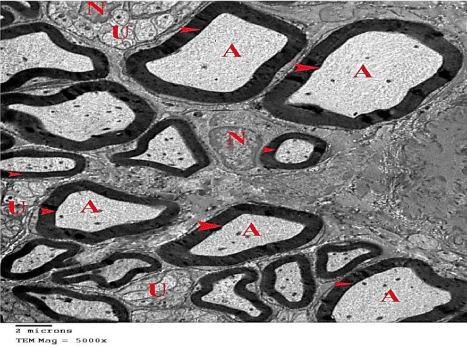
Figure 11: An electron micrograph of the sciatic nerve section from
G3, showing normal sciatic nerve composed of myelinated nerve fibers
(arrow heads) with their Axoplasm (A). Unmyelinated nerve fibers (U) and
Euchromatic Nuclei of the Schwann cell (N) are also shown. (TEM x 5000).
(iv) Cisplatin group (G4)
H&E stained sections revealed that 100% of animals were affected. The affection was in the form of disorganization and/ or shrinkage of the bundle of the myelinated nerve fibers that have obliterated myelin spaces in nearly all of them. Loss of axons was also demonstrated in 90% of the animals. Focal loss of endoneurium was also seen (Table 1) (Figure 12).
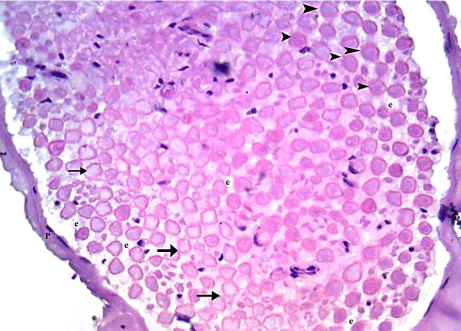
Figure 12: A transverse section of the sciatic nerve from G4, showing a
shrinked bundle surrounded by thin Perineurium (P). It contains disorganized
nerve fibers that show obliteration of myelin spaces (arrow heads) and loss
of axons (arrows) with Focal loss of Endoneurium (e) is also shown. (H&E
x 400).
In Toluidine blue semithin sections, some of the disorganized myelinated nerve fibers showed markedly thickened myelin, while others showed infoldings and outfoldings of myelin giving a starshaped appearance. Myelin fragments in the axoplasm of some nerve fibers were also seen (Figure 13).
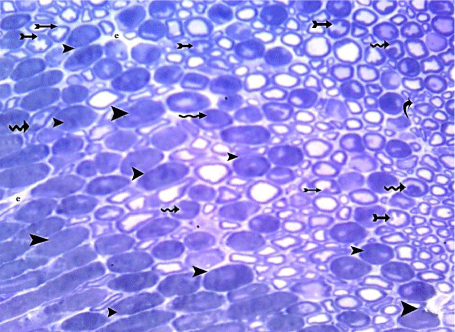
Figure 13: A semithin section of sciatic nerve from G4 showing markedly
thickened myelin (arrow heads), infoldings and outfoldings of myelin giving
a star-shaped appearance (tailed arrows) and some nerve fibers containing
myelin fragments (wavy arrows).(Toluidine blue x 400).
Transmission Electron Microscopic (TEM) confirmed the marked damage caused by cisplatin. The myelinated nerve fibers were disorganized. Some of them showed extensive splitting of their myelin sheaths while others showed marked thickening with no apparent axoplasm. Also, multiple vacuoles were seen within myelin sheaths that disrupted the axoplasm. Extensive infolding and outfolding caused severe compression of the axoplasm that occasionally contained myelin fragments. Additionally, vacuolation of axoplasm, focalloss of endoneurium and vacuolated mitochondria were also observed. Meanwhile; unmyelinated nerve fibers and euchromatic nuclei of Schwann cells appeared normal (Figures 14,15,16,17 and 18).
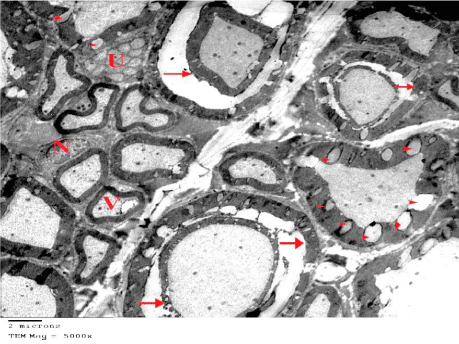
Figure 14: An electron micrograph of sciatic nerve section from G4 showing
disorganized myelinated nerve fibers. Some of them show extensive splitting
(arrows) while others have multiple vacuoles of their myelin sheaths (arrow
heads). Vacuolation (V) of axoplasm is occasionally shown. Unmyelinated
nerve fibers (U) and Euchromatic Nuclei (N) of Schwann cells appear normal.
(TEM x 5000).
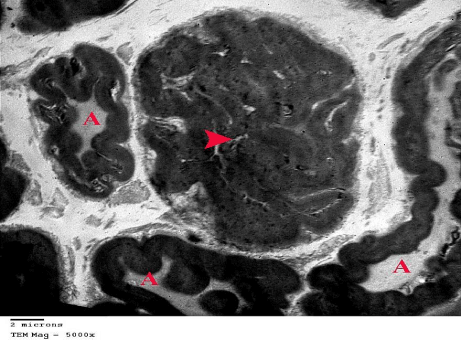
Figure 15: An electron micrograph of sciatic nerve section from G4 showing
multiple infoldings and outfoldings of some myelinated nerve fibers with
compression of their Axoplasm (A). The central myelinated nerve fiber (arrow
head) is extensively folded with no apparent axoplasm. (TEM x 5000).

Figure 16: An electron micrograph of sciatic nerve section from G4 showing
myelinated nerve fibers with markedly thickened myelin sheaths with no
apparent axoplasm (arrows). Infolding (wavy arrow) of the myelin sheath and
myelin fragments (arrow heads) in the axoplasm are also shown. (TEM x
5000).

Figure 17: An electron micrograph of the sciatic nerve section from G4
showing disorganized myelinated nerve fibers with extensive myelin splitting
into an inner thin part (tailed arrow) that surrounds the axoplasm (A) and
outer thick part that also shows focal loss of myelin (arrow heads). The space
between the two parts contains fragmented myelin (wavy arrow). Focal loss
of the Endoneurium (E) is also shown. (TEM x 15000).
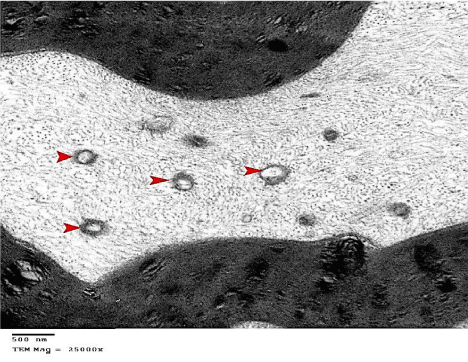
Figure 18: An electron micrograph of the sciatic nerve section from
G4 showing myelinated nerve fibers with axoplasm that contains many
vacuolated mitochondria (arrow heads). (TEM x 25000).
(v) Cisplatin + Cerebrolysin Group (G5)
H & E stained sections showed evidence of marked protection to the damaged sciatic nerves. Myelin spaces were well organized in 82% of animals, while the 18% only were still having disorganized nerve fibers with obliterated myelin spaces. No axon loss was detected in this group (Table 1) (Figure 19).
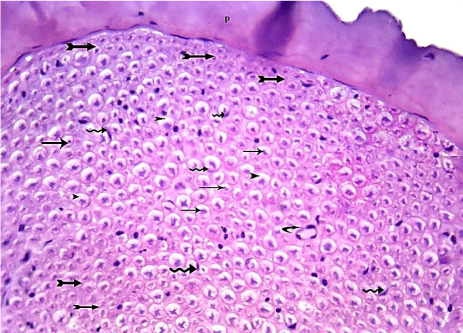
Figure 19: A transverse section of the sciatic nerve from G5 showing nearly
normal appearance of many nerve fibers that contains central axons (arrows)
surrounded by almost normal myelin spaces (arrow head) and crescentshaped
nuclei of Schwann cells (wavy arrows). Some nerve fibers are still
showing obliteration of myelin spaces (tailed arrows). Blood vessel is also
shown (curved arrow). (H&E x 400).
In Toluidine blue semithin sections, the appearance of myelinated nerve fibers was more or less normal, apart from few nerve fibers which showed minimally thickened myelin and/ or have star- shaped axoplasm (Figure 20).
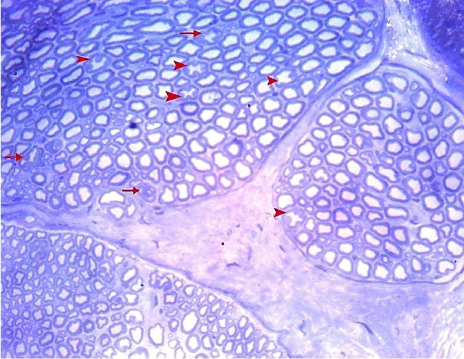
Figure 20: A semithin section of the sciatic nerve from G5 showing
myelinated nerve fibers which almost have nearly normal appearance. Few
nerve fibers show minimally thickened myelin (arrows) and/ or have starshaped
axoplasm (arrow heads). (Toluidine blue x 400).
Transmission Electron Microscopic (TEM) results showed nearly normal appearance of the myelinated nerve fibers with narrow Schmidt-Lanterman clefts and normal mitochondria. Unmyelinated nerve fibers and euchromatic nuclei of Schwann cells also exhibited normal appearance. Few nerve fibers were still showing mild myelin splitting that disrupt the axoplasm, shrunken axoplasm and myelin fragments (Figures 21,22 and 23).
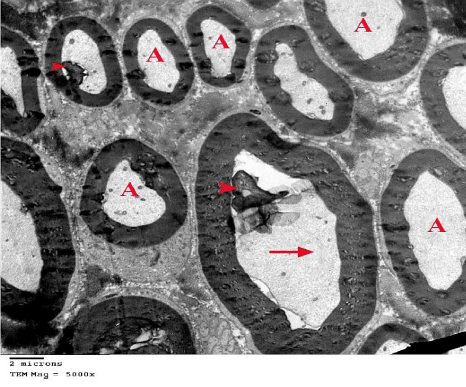
Figure 21: An electron micrograph of sciatic nerve section from G5 showing
many normal myelinated nerve fibers with homogenous Axoplasm (A), and
others with shrunken axoplasm (arrow) and myelin fragments (arrow heads).
(TEM x 5000).

Figure 22: An electron micrograph of sciatic nerve section from G5 showing
myelinated nerve fibers, containing homogenous axoplasm (A), with narrow
Schmidt-Lanterman Clefts (C). The Endoneurium (E) between the nerve
fibers is also shown. (TEM x 15000).
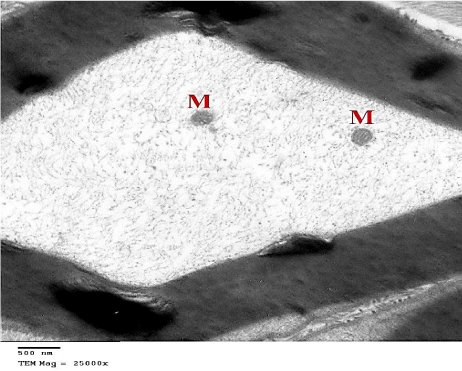
Figure 23: An electron micrograph of sciatic nerve section from G5 showing
myelinated nerve fiber with its axoplasm containing intact Mitochondria (M).
(TEM x 25000).
(vi) Cisplatin + Gingko biloba (G6)
H & E stained sections showed evidence of limited protection to the damaged sciatic nerve. Myelin spaces were well organized in 55% of animals, while the rest of them (45%) were still having disorganized nerve fibers with obliterated myelin spaces. Nerve fibers with preserved axons were demonstrated in 63% of animals, while loss of some axons was detected in the rest of them (37%) (Table 1) (Figure 24).
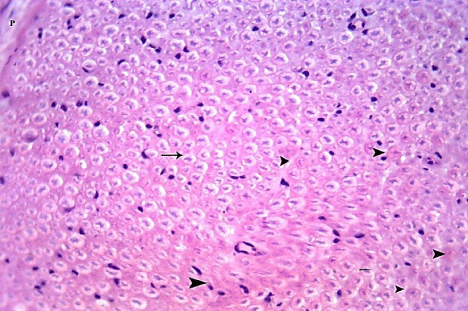
Figure 24: A transverse section in the sciatic nerve from G6 showing some
nerve fibers with nearly normal appearance (arrow) and other fibers still show
obliteration of myelin spaces (arrow heads). (H&E x 400).
In Toluidine blue semithin sections, some myelinated nerve fibers were normal while others revealed thickened myelin and/ or star like appearance. Focal loss of endoneurium was also still seen (Figure 25).
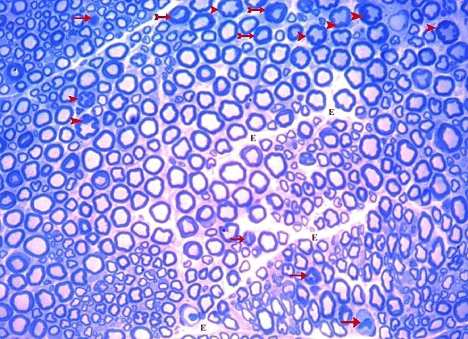
Figure 25: A semithin section of the sciatic nerve from G6 showing some
normal myelinated nerve fibers. It also shows many nerve fibers with star-like
appearance (arrow heads), minimally thickened (tailed arrow) or markedly
thickened myelin (arrows). Focal loss of Endoneurium (E) is also shown.
(Toluidine blue x 400).
Transmission Electron Microscopic (TEM) examination confirmed the limited protection of sciatic nerve as it showed normal appearance of some myelinated fibers, while many other fibers showed myelin splitting, vacuolation and Myelin fragments in the axoplasm. Focal loss of endoneurium was also noticed (Figures 26,27 and 28).
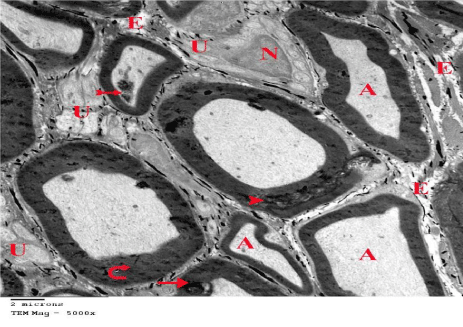
Figure 26: An electron micrograph of section from G6 showing some normal
myelinated nerve fibers with homogenous Axoplasm (A). Unmyelinated
(U) nerve fibers and Euchromatic Nuclei (N) of Schwann cells also appear
normal. Some myelinated nerve fibers are thickened (curved arrows) and still
showing minimal myelin splitting (arrow heads) that disrupts the axoplasm
(arrow). Myelin fragments in the axoplasm (tailed arrows) and focal loss of
Endoneurium (E) are also shown. (TEM x 5000).
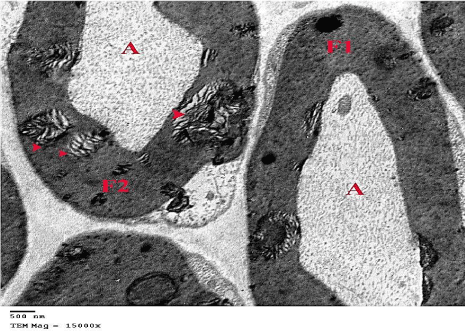
Figure 27: An electron micrograph from G6 showing a normal myelinated
nerve fiber (F1) side by side to a damaged fiber (F2) showing separation of
myelin (arrow heads). (TEM x 15000).
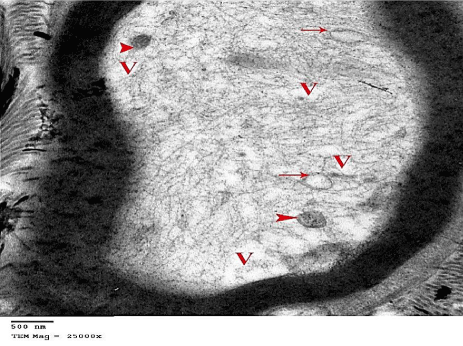
Figure 28: An electron micrograph from G6 showing myelinated nerve fibers
with Vacuolated (V) axoplasm that contains mitochondria with no apparent
cristea (arrow heads). Some vacuolated mitochondria (arrow) are also
shown. (TEM x 25000).
Discussion
Cisplatin is one of the most remarkable successes in ‘the war on cancer’. It is one of the platinum-containing compounds and considered one of the most effective and potent anticancer drugs used in the treatment of a wide variety of both pediatric and adult malignancies. However, the therapeutic use of cisplatin is limited by its serious dose-limiting side effects including cisplatin-induced peripheral neuropathy [3,25,26]. This neuropathy is mainly sensory and can be extremely painful causing debilitating symptoms that significantly affect the patient’s quality of life. This may require modifications in chemotherapy treatment regimen either by dose reduction or discontinuation of cisplatin treatment, which reduces its efficacy [27-29]. This necessitates the search for protective agents that could prevent or at least ameliorate cisplatin-induced peripheral neuropathy, without interfering with its antineoplastic activity. This study was designed to investigate the toxic effects of cisplatin on sciatic nerve (as a peripheral nerve) and the possible neuroprotective effects of cerebrolysin versus Gingko biloba against cisplatin-induced peripheral neuropathy, using light and transmission electron microscopes.
In the present work, cisplatin treated animals received a dose of2 mg/kg BW IP, twice a week for 4 weeks to induce peripheral neuropathy. This model is well documented and has been widely used in pre-clinical studies on cisplatin- induced neuropathy [30]. The sciatic nerve, as the thickest and largest nerve in the body, has a great sensitivity to neurotoxic effects of cancer chemotherapy probably due to its high metabolic requirements [31,32].
During early days of treatment; signs of general toxicity appeared and manifested in body weight reduction in rats of this group compared to control group. This was in agreement with results of Ozturk et al. [33]; who investigated the neurotoxic effect of cisplatin in mice.
Examination of H&E stained sections in cisplatin group showed marked disorganization and/or shrinkage of myelinated nerve fibers with obliterated myelin spaces in nearly all fibers and loss of axons in many fibers. Toluidine blue semithin sections furthermore showed marked thickening and infoldings and outfoldings of myelin giving the fibers a star-shaped appearance. Myelin fragments in the axoplasm of some nerve fibers were also observed. This was confirmed ultra structurally, as TEM examination showed marked thickening, extensive splitting and infoldings and outfoldings of the myelin sheaths. Severe compression of the axoplasm that occasionally contained myelin fragments was also observed. Vacuolated mitochondria and multiple vacuoles that disrupted the axoplasm were also noticed. Kamisli et al. [34,35] investigated the protective effect of hesperidin (a citrus flavonoid) and fish oil, respectively, against cisplatin-induced oxidative stress in sciatic nerve. They found axonal degeneration, which was attributed to the imbalance between the oxidant and antioxidant status induced by cisplatin. Kamel, [36] using transmission electron microscope, demonstrated extensive splitting and thickening of myelin, axoplasmic vacuolations and myelin fragmentation in the myelinated nerve fibers of sciatic nerve. This researcher investigated the protective effect of alpha-lipoic acid against the neurotoxic effect of cypermethrin (insecticide) on sciatic nerve of albino rats. He reported that myelin splitting could be related to accumulation of edema fluid. This can also explain the cause of obliterated spaces of myelin which appeared in H&E sections. The author added that the myelin fragment denoted Wallerian degeneration. It was reported that the peripheral nerve degeneration is generally accepted as an underlying cause for the development of chemotherapy-induced peripheral neuropathy [37].
In our study, vacuolated mitochondria were demonstrated in cisplatin group. Ali, [38], who performed a biochemical and ultra structural study to investigate the protective effect of ginger extract against cisplatin-induced hepatotoxicity in adult male albino rats, found swollen or fused mitochondria in the hepatocytes of rats. This discrepancy may be due to different dose (3.3 mg/ kg BW) and duration (3 consecutive days) of cisplatin administration. It was reported that direct mitochondrial damage by cisplatin occurred due to formation of adducts between cisplatin and mitochondrial DNA. This could inhibit mitochondrial DNA replication and mitochondrial RNA transcription [39].
In the present work, the unmyelinated nerve fibers were unaffected in cisplatin group. This can be explained by the findings of Carozzi et al. [40] and Hwang et al. [41] who reported that the neurotoxic effect of cisplatin in human and animals occurred via induction of lipid peroxidation. Accordingly, cisplatin might tend to concentrate in myelin sheaths affecting its lipid nature away from the unmyelinated ones. Additionally, normal appearance of Schwann cells in rats of cisplatin- treated group might be due to limited oxidative effect of cisplatin on these cells. This is supported by the suggestion of Zheng et al. [42] who reported that the mitochondria of the Schwann cell are relatively spared from adducts to platinumcontaining chemotherapeutic agents.
In the present study, concomitant administration of cerebrolysin with cisplatin resulted in less degenerative changes of sciatic nerve. H&E stained sections showed well organized myelin spaces of sciatic nerve in many animals. Few animals were still having disorganized nerve fibers with obliterated myelin spaces. No axon loss was detected in this group. There was a statistically significant decrease regarding obliteration of myelin spaces, loss of axons and vacuolar degeneration compared to cisplatin group. Also, Toluidine blue semithin sections showed almost normal appearance of myelinated nerve fibers apart from few nerve fibers which showed minimally thickened myelin and/ or have star- shaped axoplasm. Ultrastructurally, the neuroprotective effect of cerebrolysin was confirmed. There was nearly normal appearance of the myelinated nerve fibers with narrow Schmidt- Lanterman clefts and normal mitochondria. On the other hand, few nerve fibers were still showing mild myelin splitting, shrunken axoplasm, myelin fragments and few degenerated mitochondria. Up to our knowledge, there are no previous histological studies demonstrated the neuroprotective effect of cerebrolysin against cisplatin- induced peripheral neuropathy. However, Shchudlo et al. [43] investigated the effect of cerebrolysin on the dynamics and long-term results of peripheral nerve regeneration after transaction and microsurgical suturing. They found that cerebrolysin has a stimulating effect on the regenerative growth and differentiation of nerve fiber axial cylinders, vascularization of the distal segment of regenerating nerve and the trophic condition of myelin-forming Schwann cells. They noticed that its effect persisted for up to 10.5 months after the end of paraneural injection course. Additionally, Zangiabadi et al. [44] concluded that cerebrolysin has a significant efficacy in improvement of nerve conduction velocity of peripheral nerves in experimentally-induced diabetes. Moreover, cerebrolysin had neuroprotective effects on the survival of retinal ganglion cells and visual function after optic nerve crush in rat model [45].
In our study, the nearly normal appearance of axoplasmic mitochondria indicated that cerebrolysin could restore and/or enhance normal mitochondrial function, which is considered the primary target of cisplatin oxidative cytotoxicity. Accordingly, cerebrolysin reversed almost all pathological changes induced by cisplatin in sciatic nerve of our study.
Also in the present work, concomitant administration of Gingko biloba with cisplatin resulted in less degenerative changes of sciatic nerves compared to cisplatin group, however the protection was less than that of cisplatin + cerebrolysin group. This was evident by examination of H&E and Toluidine blue semithin sections which showed loss of axons in some nerve fibers and focal loss of endoneurium. These results were confirmed ultrastructurally by TEM that furthermore showed partial improvement of myelinated nerve fibers. Our results were in accordance with Ozturk et al. [33] who found that Gingko biloba provided partial protection against cisplatin- induced neurotoxicity in mice. They attributed this neuroprotection to its antioxidant and anti-inflammatory activities.
Also in this group, TEM showed persistent degeneration of all axoplasmic mitochondria, which can be explained by partial uncoupling of mitochondria from oxidative phosphorylation by Gingko biloba. This was suggested by Bernatoniene et al. [46] who assessed the influence of Gingko biloba in rats exposed to 70% ethanol and evaluated the functions of isolated heart mitochondria under normal and ischemic conditions.
Conclusion
Cisplatin induced histopathological changes in myelinated nerve fibers of the sciatic nerve. Cerebrolysin had more neuroprotective effect than Gingko biloba against cisplatin-induced peripheral neuropathy.
References
- Clark MA, Finkel R, Rey JA, Whalen K, Harvey RA. Anticancer drugs. In: Lippincott’s Illustrated Review. Pharmacology. 5th edn. 2012; 39: 507-508.
- Erbas O, Akman L, Yavasoglu A, Terek MC, Akman T, Taskiran D. Oxytocin improves follicular reserve in a cisplatin-induced gonadotoxicity model in rats. Biomed research international volume. 2014.
- Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic Acid Phenethyl Ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol. 2010; 62: 45-52.
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012; 31: 1869-1883.
- Salman M, Naseem I. Riboflavin as adjuvant with cisplatin: study in mouse skin cancer model. Front Biosci (Elite Ed). 2015; 7: 242-254.
- Balayssac D, Ferrier J, Descoeur J, Ling B, Pezet D, Eschalier A, et al. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf. 2011; 10: 407-417.
- Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013; 9: 25-33.
- Miltemburg NC. Boogerd W. Chemotherapy-induced neouropathy: A comprehensive survey. Cancer Treat Rev. 2014; 40: 872- 882.
- Rose N, Clarence D. Management of Chemotherapy-Induced Peripheral Neuropathy. US Pharm. 2015; 40: HS5-HS10.
- Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rørth M, Krarup C. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain. 2007; 130: 1076-1088.
- Abd El-Ghany RM, Sharaf NM, Kassem LA, Mahran LG, Heikal OA. Thymoquinone triggers anti-apoptotic signaling targeting death ligand and apoptotic regulators in a model of hepatic ischemia reperfusion injury. Drug Discov Ther. 2009; 3: 296-306.
- Kassem LA, Yassin NA. Role of erythropoeitin in prevention of chemotherapy-induced peripheral neuropathy. Pak J Biol Sci. 2010; 13: 577-587.
- LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology. 2013; 37: 231-239.
- Ghirardi O, Vertechy M, Vesci L, Canta A, Nicolini G, Galbiati S, et al. Chemotherapy-induced allodinia: neuroprotective effect of acetyl-L-carnitine. In Vivo. 2005; 19: 631-637.
- Lin PC, Lee MY, Wang WS, Yen CC, Chao TC, Hsiao LT, et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer. 2006; 14: 484-487.
- Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008; 214: 276-284.
- Abdelaziz OS, Elbanna YM, Elnaggar AM, Mourad GM, Mahmoud SA. Effects of neuropeptide derivative FPF 1070 (Cerebrolysin) administration on experimental acute spinal cord injury: A histological study. The Egyptian journal of Histology. 2005; 28: 35-48.
- Masliah E, Díez-Tejedor E. The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs Today (Barc). 2012; 48 Suppl A: 3-24.
- Chen C, Wei S, Tsaia S, Chen X, Cho D. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double- blind, placebo- controlled, randomized study. Br J Neurosurgery. 2013; 27: 803-807.
- Rojas P, Montes P, Rojas C, Serran o-Garcia, Rojas-Castaneda J. Effect of phytopharmaceutical medicine, Gingko biloba extract 761, in an animal model of Parkinson’s disease: therapeutic perspectives. Nutrition. 2012; 28: 1081-1088.
- Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013; 52: 727-749.
- Franke AG, Heinrich I, Lieb K, Fellgiebel A. The use of Ginkgo biloba in healthy elderly. Age (Dordr). 2014; 36: 435-444.
- Tan MS, Yu JT, Tan CC, Wang HF, Meng XF, Wang C, et al. Efficacy and adverse effects of ginkgo biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2015; 43: 589-603.
- Bozzola J, Russell L. Electron microscope. In: Principles and Techniques for biologists. 2nd Edn. Jones and Bartlett Publishers. 1999; 542.
- Hardman J, Limbird L, Gilman AG. Antineoplastic agents. Goodman, Gilman, editors. In: The pharmacological basis of therapeutics. 10th Edn. 2011; 52: 1432- 1434.
- Araujo J, Sampaio A, Venosa A, Pires C. The potential use of melatonin for preventing cisplatin ototoxicity: An insight for a clinical approach. Advances in otolaryngology. 2014.
- Gopal KV, Wu C, Shrestha B, Campbell KC, Moore EJ, Gross GW. d-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicol Teratol. 2012; 34: 495-504.
- Han Y, Smith MT. Pathobiology of cancer Chemotherapy-Induced Peripheral Neuropathy (CIPN). Front Pharmacol. 2013; 4: 156.
- Bianchi R, Brines M, Lauria G, Savino C, Gilardini A, Nicolini G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental Cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006; 12: 2607-2612.
- Mironov SL. ADP regulates movements of mitochondria in neurons. Biophys J. 2007; 92: 2944-2952.
- Sinnatamby CS. Last’s anatomy. Regional and applied. 12th Edn. London: Elsevier Health Sciences. UK. 2011.
- Gutierrez-Gutierrez G, Sereno M, Miralles A, Casado-Saenz E, Gutierrez-Rivas E. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol. 2010; 12: 81-91.
- Oztürk G, Anlar O, Erdoğan E, Kösem M, Ozbek H, Türker A. The effect of Ginkgo extract EGb761 in cisplatin-induced peripheral neuropathy in mice. Toxicol Appl Pharmacol. 2004; 196: 169-175.
- Kamisli S, Ciftci O, Cetin A, Kaya K, Kamisli O, Celik H. Fish oil protects the peripheral and central nervous systems against cisplatin-induced neurotoxicity. Nutr Neurosci. 2014; 17: 116-126.
- Kamisli S, Ciftci O, Kaya K, Cetin A, Kamisli O, Ozcan C. Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol Ind Health. 2015; 31: 841-851.
- Kamel A. Histologic study on the protective effect of alpha- lipoic acid in sciatic nerve neurotoxicity induced by cypermethrin in albino rats. The Egyptian Journal of Histology. 2011; 34: 218-230.
- Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012; 59: 3-9.
- Ali DA. Biochemical and ultrastructural studies on the protective effect of ginger extract against cisplatininduced hepatotoxicity in adult male albino rats. The Egyptian Journal of Histology. 2011; 34: 231- 238.
- Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011; 41: 661-668.
- Carozzi VA, Marmiroli P, Cavaletti G. The role of oxidative stress and anti-oxidant treatment in platinum-induced peripheral neurotoxicity. Curr Cancer Drug Targets. 2010; 10: 670-682.
- Hwang SL, Shih PH, Yen GC. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012; 60: 877-885.
- Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011; 232: 154-161.
- Shchudlo NA, Shchudlo MM, Borisova IV. [The effect of cerebrolysin on the regeneration of the peripheral nerve depending on the scheme of paraneural administration]. Zh Nevrol Psikhiatr Im S S Korsakova. 2013; 113: 76-80.
- Zangiabadi N, Mohtashami H, Shabani M, Jafari M. Neuroprotective effects of cerebrolysin on diabetic neuropathy: A study on male rats. J Diabetes Metab. 2014; 5: 4.
- Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK. Protective effects of cerebrolysin in a rat model of optic nerve crush. Kaohsiung J Med Sci. 2014; 30: 331-336.
- Bernatoniene J, Majiene D, Peciura R, Laukeviciene A, Bernatoniene R, Mekas T, et al. The effect of Ginkgo biloba extract on mitochondrial oxidative phosphorylation in the normal and ischemic rat heart. Phytother Res. 2011; 25: 1054-1060.