
Review Article
Austin Neurosurg Open Access. 2016; 3(1): 1043.
Image Guided Spine Surgery: Available Technology and Future Potential
Karhade AV, Vasudeva VS, Pompeu YA, Lu Y*
Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, USA
*Corresponding author: Yi Lu, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA
Received: October 26, 2015; Accepted: January 18,2016; Published: January 19, 2016
Abstract
Image-guided navigation systems for spinal surgery have evolved tremendously since they first became available in the 1990s. This technology, borrowed from cranial navigation systems, was initially difficult to use during spinal surgery due to intraoperative shift of spinal anatomy and of the inability to use skin and surface landmarks for registration. Spinal imaging systems include C-arm fluoroscopy, preoperative Computed Tomography (CT) based navigation, 2D fluoroscopy based navigation, and more recently, cone beam CT based navigation and intraoperative CT based navigation. Although the more primitive intraoperative imaging systems are relatively inexpensive and widely available, they require additional pre-operative preparation time and re-registration at each level for multiple level surgeries. Furthermore, they cannot produce axial reconstructions and expose the surgeon and operating room personnel to radiation. Newer imaging techniques such as cone beam CT based navigation and intraoperative CT based navigation allow for automatic registration, three-dimensional, multi-planar reconstructions, extended scan volume, and eliminate the need to obtain specialized pre-operative imaging for registration. Intraoperative image-guided spinal navigation has been shown to be a useful adjunct for spinal surgeons especially during the placement of spinal implants. This technology is particularly useful during minimally invasive spine procedures where direct visualization of the spinal anatomy is often not possible. We believe that ongoing advances in intraoperative image acquisition and navigation will lead to decreased complication rates and improved outcomes in the future.
Keywords: Image guidance; Neuronavigation; Spine; Spinal surgery; C-arm fluoroscopy; Preoperative CT; 2D fluoroscopy; Cone beam CT; Intraoperative CT
Introduction
The past three decades of image-guided spine surgery have witnessed the development of multiple modalities for intraoperative imaging and navigation. The ultimate utility of these technologies depends on a critical appraisal of the unique advantages and disadvantages of each system. New generation tools such as cone beam CT and intraoperative CT have made tremendous improvements over initial technologies. For example, Barsa et al. placed 571 spinal implants with 99.13% accuracy [1,2] and Tormenti et al. placed 164 thoracolumbar pedicle screws using intraoperative CT with 1.2% pedicle breach compared to 5.2% with standard fluoroscopy [2,3]. Going forward, novel systems that optimize neuronavigation for minimally invasive spine surgery will lead to, in the short term, decreased complication rates and improved outcomes, and, in the long term, more innovative surgical procedures for existing problems.
Intraoperative image-guided navigation was first introduced to spine surgery in the mid-1990s [2,4]. The technology behind this advancement was initially developed for intracranial neurosurgery [2,5,6]. However, the application of this technology was more challenging for spinal neurosurgeons due to the inherent complexity of the anatomy of the spinal column. For example, the position of the brain within the skull is relatively constant, so cranial neurosurgeons were able to perform registration for intraoperative navigation based on high resolution imaging studies that were obtained preoperatively. For spinal neurosurgeons this proved difficult since the configuration of the spinal column could shift when the patient was positioned for surgery [7-10]. Additionally, skin surface landmarks were reliable for point and surface matching registration techniques during cranial neurosurgery. Conversely, in the spine, the skin and underlying soft tissue are mobile relative to the spinal column and it was therefore necessary to use bony landmarks for registration that required an extensive and meticulous surgical exposure [2,7-10].
Although the use of intraoperative navigation was not initially compatible with spinal anatomy, there was great demand for this technology amongst spine surgeons who felt that navigation would be especially useful in situations where spinal implants were placed without direct visualization. For example, the placement of pedicle screws is a common surgical procedure during which the surgeon must place an implant based on anatomical landmarks with minimal feedback to truly know that the trajectory of the screw will lie entirely within the confines of the pedicle [11]. This has led to rates of pedicle screw misplacement that have been reported as high as 40% in the lumbar spine and 55% in the thoracic spine [12,13]. Although these numbers are clearly overestimates by today’s standards, they illustrate that the misplacement of hardware can occur even in good hands and has been a longstanding concern for spine surgeons. In addition, with the introduction of minimally invasive surgical techniques for spine surgery, surgeons were trying to perform larger operations through smaller skin incisions and with less bony exposure. Intraoperative navigation had obvious utility in these procedures and motivated spine surgeons to modify and adopt navigation technology.
At our institution, we do not routinely use advanced intraoperative image navigation for all spinal surgeries. Rather, we consider using this technology when it is available in cases where there is unusual anatomy or when a difficult surgical approach is used.
Systems Used for Image-Guided Navigation
The technology used to acquire imaging for intraoperative navigation has evolved from the discovery of X-rays in the late 19th century to the highly sophisticated intraoperative Computed Tomography (CT) based navigation tools used today [14]. The range of available technologies includes C-arm fluoroscopy, preoperative CT based navigation, 2D fluoroscopy based navigation, cone beam CT based navigation, and intraoperative CT based navigation. Aside from fluoroscopy, these imaging modalities implement the basic steps of image acquisition, registration to patient anatomy, processing, and navigation [2,14,15].
C-Arm Fluoroscopy
C-arm fluoroscopy is the most widely available mode of intraoperative image acquisition and allows for the rapid and serial visualization of 2-dimensional images in real time. This imaging modality is a quick and effective way to determine the correct level of surgery. During minimally invasive procedures requiring biplanar fluoroscopy, two c-arms must be positioned into true Anteroposterior (AP) and lateral projections. True AP calibrations require visualization of the superior endplate as a single line, the pedicle shadows caudal to the superior endplate, and the spinous process shadow at the midpoint of the pedicle shadows. Lateral calibrations require visualization of both the superior endplate as a single line and the superimposition of the left and right pedicle shadows onto the posterior cortex of the vertebral body as a single line. Three-dimensional anatomy must be indirectly inferred from the 2-D images.
The major advantages of C-arm fluoroscopy are its low cost and widespread availability (Table 1). C-arm fluoroscopy also provides imaging in real time. The major disadvantages of C-arm fluoroscopy are that it exposes the surgeon and Operating Room (OR) personnel to radiation, it is cumber some to obtain images in multiple planes simultaneously, there are ergonomic challenges associated with the C-arm’s positioning, and the inability to visualize images in the axial plane [2,10,16,17].
Imaging modality
Benefits
Can be used with intraoperative navigation system?
Requires additional pre-operative imaging?
Need for re-registration for multiple level surgeries?
Ability to visualize images in axial plane?
C-arm Fluoroscopy
Only truly real-time imaging modality
No
No
N/A
No
Preoperative CT
Preoperative CT may be coupled to intraoperative navigation system
Yes
Yes
Yes
Yes
2D Fluoroscopy
Navigation may be performed using images acquired intraoperatively in the surgical position. Automatic registration is possible
Yes
No
No
No
Cone Beam CT
3-dimensional images may be acquired during surgery and used for navigation
Yes
No
No
Yes
Intraoperative CT
Higher resolution imaging and extended scan volume when compared to cbCT
Yes
No
No
Yes
Table 1: Characteristics, advantages, and disadvantages of available imaging modalities.
Preoperative CT Based Navigation
Preoperative CT based navigation was the first available mode of intraoperative navigation. A navigation workstation takes input from a 2-dimensional thin-cut CT through the region of interest that is obtained prior to surgery and creates a virtual three-dimensional reconstruction that can be used for surgical planning and to simulate the placement of implants [2,4,18,19]. Anatomical landmarks that will be used for registration are selected on the preoperative reconstruction [2,8,18,19]. After the surgical exposure is complete, a Dynamic Reference Base (DRB) is attached to a fixed anatomical location on the patient [18]. Then, using either point-matching or surface-matching techniques, preoperative imaging is registered to the patient’s anatomy [18,19]. To account for shifting anatomy during surgery, each level must be registered separately.
Unlike conventional c-arm fluoroscopy, preoperative CT based navigation systems allowed surgeons to track the position of surgical instruments in space and to plan the placement of hardware implants on virtual 3D multi-planar image reconstructions (Table 1) [18,19]. The disadvantages of this technology included increased radiation exposure to the patient, and additional preoperative preparation time [10]. In addition, extensive bony exposure is required for adequate registration and it may be difficult to identify landmarks for registration in patients with prior laminectomies [10,18]. Because each segment of the vertebral column could shift in between the preoperative CT and positioning in the OR, re-registration was required for each level in multi-level surgeries and this proved to be time consuming and tedious [19].
2D Fluoroscopy Based Navigation
In 2D fluoroscopy based navigation, successful registration does not rely on pre-operative imaging; this overcomes the principle challenge of preoperative CT based navigation [4,20]. A navigation system is oriented to the geometric configuration of the c-arm [2,10,21]. The navigation system recognizes a calibration target on the c-arm and registers AP and lateral fluoroscopic images from a DRB attached to the patient [21]. The camera of the navigation system recognizes surgical instruments within the scanned area [21]. The navigation system outputs the presence of instruments by superimposing the images of the instruments onto the fluoroscopic images of the patient anatomy (Figure 1) [21].
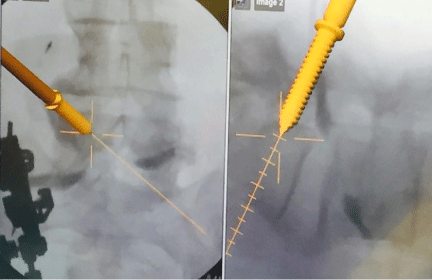
Figure 1: Intraoperative image guidance using a 2D fluoroscopy based navigation system showing the real-time placement of pedicle screws.
Advantages of 2D fluoroscopy based navigation include reduction in anatomic shift error, automatic registration, reduced patient radiation exposure in comparison to preoperative CT based navigation, and improved ergonomics for multi-planar imaging due to greater c-arm mobility in comparison to conventional c-arm fluoroscopy [4,21]. Furthermore, c-arm fluoroscopy is readily available to check real-time images whenever they are needed. This is particularly useful during placement of hardware such as pedicle screws, interbody cages, and when manipulating rods for percutaneous pedicle screws (Figure 2). The disadvantages of 2D fluoroscopy include lack of three-dimensional imaging and axial reconstructions, narrow field of view, and difficulty in imaging obese and osteopenic patients [2,10,21,22].
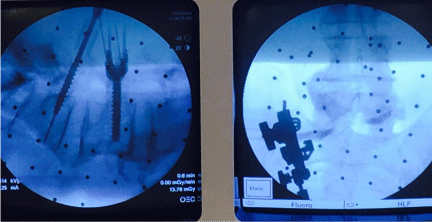
Figure 2: A significant benefit of using 2D fluoroscopy based navigation systems is the quick availability of the c-arm to check the real-time images during placement of hardware, such as pedicle screws shown above.
Cone Beam CT Based Navigation
Cone beam CT (cbCT) is another imaging modality used for obtaining imaging for intraoperative navigation. A high-resolution three-dimensional view is obtained from the “spin” of a cone-shaped X-ray beam about the patient. The spin generates multiple images that are processed into the three-dimensional dataset [2,4,10,21]. Analogous to the tracking of the c-arm and the patient anatomy in 2D fluoroscopy based navigation; automatic registration is achieved by tracking the locations of the X-ray source and DRB within the cbCT scanned area [4].
Like 2D fluoroscopy based navigation, cbCT based navigation systems perform automatic registration and eliminate the error caused by anatomic shift by scanning the patient in the surgical position (Table 1) [22]. Cone-beam CT systems also produce three-dimensional and multi-planar imaging and provide better visualization of bony structures in obese and osteopenic patients [2,18,21]. The main disadvantage of cbCT compared to intraoperative CT based navigation is that only several levels can be visualized at a time depending on the scan volume per “spin”. For large deformity surgeries where the entire surgical site cannot be included within one “spin”, the cbCT device must be re-centered [22].
The most widely used cone beam CT based navigation system is the Medtronic O-arm system. Prior to the start of the surgery, after the patient is positioned, a dynamic reference base is connected to a pin inserted into the iliac crest (Figure 3) or a spinous process clamp. O-arm machine is then brought into the field and closed around the OR table to change the configuration from a “C” to an “O”. Images are then acquired by performing a “spin” of the O-arm. Following this, the O-arm is then generally removed from the surgical field due to its bulky size. Image guided surgical navigation can then be performed based on the 3-D reconstructed images from the O-arm. At the end of surgery, if the accuracy of the hardware needs to be checked, the O-arm need to be brought into the field for the 2nd time and another “spin” is performed. The benefit is that the axial reconstituted films can be obtained right away to confirm the accurate placement of the hardware in all three planes (axial, coronal and sagittal) (Figure 4).
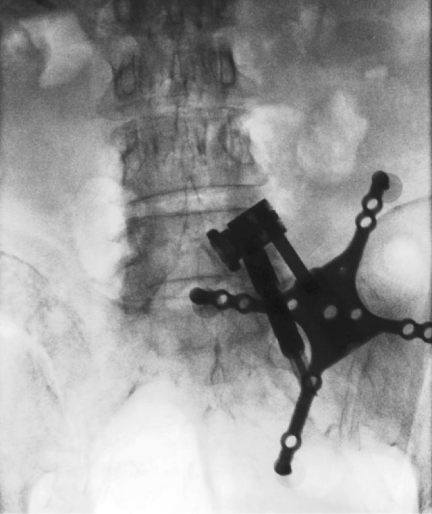
Figure 3: AP fluoroscopic image showing the placement of a dynamic reference base for intraoperative navigation docked to a pin inserted into the
iliac crest.
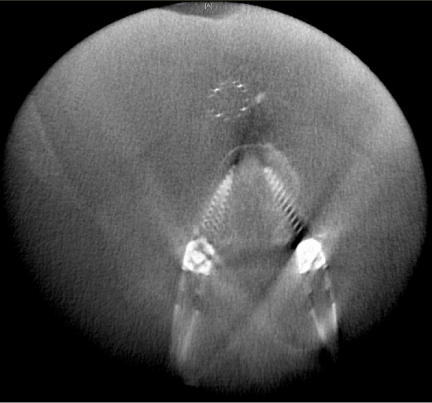
Figure 4: Axial reconstruction created intraoperatively with O-arm (Medtronic) confirming appropriate positioning of percutaneous lumbar pedicle screws.
Intraoperative CT Based Navigation
Intraoperative CT (iCT) builds on the principles of cbCT based navigation but eliminates the need to re-center the machine due to its larger field of view. This navigation technology uses a portable CTs canner that translates in the rostral to caudal axisover the patient [2,23,24]. The iCT navigation workstation registers to the patient’s anatomy and creates three-dimensional images [23]. These images are used to visualize the trajectory of surgical instruments and implants.
The major benefits of iCT based navigation systems are increased image quality and an extended scan volume which eliminates the need to re-center the device even in large deformity cases [4]. Disadvantages of iCT include high cost, inferior ergonomics compared to cbCT, increased preparation time, and less flexibility due to requirements for specialized operating tables [1].
Future Directions in Image Guided Neuronavigation
Image-guided neuronavigation has many implications for the future of spine surgery especially as minimally invasive procedures become more commonly performed. Minimally invasive spine surgery is defined as surgeries that use a minimal exposure to preserve muscular attachments and neurovascular structures resulting in less surgical pain, lower risk of infection, less blood loss, and faster recovery [18]. Because minimal exposure often also means minimal visualization, image guidance has proven to be particularly useful during these surgeries. For example, the use of image-guidance facilitates percutaneous pedicle screw placement without the need for bony exposure or direct visualization of anatomic landmarks required in conventional pedicle screw placement [18]. In addition, new trajectories for pedicle screw placement are made possible by image guidance such as medial-to-lateral pedicle screw insertion
Conclusion
Over the last several decades, multiple methods of image-guided navigation for spinal surgery have emerged. This technology has proved to be a useful adjunct during the placement of spinal implants without direct visualization. Disadvantages of early systems have been improved in newer generation technologies that allow for automatic registration, three-dimensional, multi-planar reconstructions, and extended scan volume. We expect that in the future, the use of navigation in spinal surgery will enhance the safety and efficacy of currently accepted operations and will facilitate the development of new minimally invasive techniques.
References
- Barsa P, Frohlich R, Beneš V 3rd, Suchomel P. Intraoperative portable CT-scanner based spinal navigation--a feasibility and safety study. Acta Neurochir (Wien). 2014; 156: 1807-1812
- Vasudeva V, Moses Z, Cole T, Gologorsky Y, Lu Y. Chapter 14 - Image Guidance for Spine Surgery. Golby AJ, editor. In: Image-Guided Neurosurgery. Academic Press: Boston. 2015; 325-364.
- Tormenti MJ, Kostov DB, Gardner PA, Kanter AS, Spiro RM, Okonkwo DO. Intraoperative computed tomography image-guided navigation for posterior thoracolumbar spinal instrumentation in spinal deformity surgery. Neurosurg Focus. 2010; 28: E11.
- Nottmeier EW. A review of image-guided spinal surgery. J Neurosurg Sci. 2012; 56: 35-47.
- Nolte LP, Zamorano L, Visarius H, Berlemann U, Langlotz F, Arm E, et al. Clinical evaluation of a system for precision enhancement in spine surgery. Clin Biomech (Bristol, Avon). 1995; 10: 293-303.
- Nolte LP, Visarius H, Arm E, Langlotz F, Schwarzenbach O, Zamorano L. Computer-aided fixation of spinal implants. J Image Guid Surg. 1995; 1: 88-93.
- Brodwater BK, Roberts DW, Nakajima T, Friets EM, Strohbehn JW. Extracranial application of the frameless stereotactic operating microscope: experience with lumbar spine. Neurosurgery. 1993; 32: 209-213.
- Lee TC, Yang LC, Liliang PC, Su TM, Rau CS, Chen HJ. Single versus separate registration for computer-assisted lumbar pedicle screw placement. Spine (Phila Pa 1976). 2004; 29: 1585-1589.
- Roessler K, Ungersboeck K, Dietrich W, Aichholzer M, Hittmeir K, Matula C, et al. Frameless stereotactic guided neurosurgery: clinical experience with an infrared based pointer device navigation system. Acta Neurochir (Wien). 1997; 139: 551-559.
- Holly LT. Image-guided spinal surgery. Int J Med Robot. 2006; 2: 7-15.
- Steinmann JC, Herkowitz HN, el-Kommos H, Wesolowski DP. Spinal pedicle fixation. Confirmation of an image-based technique for screw placement. Spine (Phila Pa 1976). 1993; 18: 1856-1861.
- Belmont PJ Jr, Klemme WR, Dhawan A, Polly DW Jr. In vivo accuracy of thoracic pedicle screws. Spine (Phila Pa 1976). 2001; 26: 2340-2346.
- Xu R, Ebraheim NA, Ou Y, Yeasting RA. Anatomic considerations of pedicle screw placement in the thoracic spine. Roy-Camille technique versus open-lamina technique. Spine (Phila Pa 1976). 1998; 23: 1065-1068.
- Mezger U, Jendrewski C, Bartels M. Navigation in surgery. Langenbecks Arch Surg. 2013; 398: 501-514.
- Wong KC, Kumta SM. Use of Computer Navigation in Orthopedic Oncology. Curr Surg Rep. 2014; 2: 47.
- Thambiraj S, Quraishi NA. Intra-operative localisation of thoracic spine level: a simple "'K'-wire in pedicle" technique. Eur Spine J. 2012; 21: 221-224.
- Sanders R, Koval KJ, DiPasquale T, Schmelling G, Stenzler S, Ross E. Exposure of the orthopaedic surgeon to radiation. J Bone Joint Surg Am. 1993; 75: 326-330.
- Moses ZB, Mayer RR, Strickland BA, Kretzer RM, Wolinsky JP, Gokaslan ZL, et al. Neuronavigation in minimally invasive spine surgery. Neurosurg Focus. 2013; 35: E12.
- Nottmeier EW, Crosby TL. Timing of paired points and surface matching registration in three-dimensional (3D) image-guided spinal surgery. J Spinal Disord Tech. 2007; 20: 268-270.
- Tian NF, Huang QS, Zhou P, Zhou Y, Wu RK, Lou Y, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J. 2011; 20: 846-859.
- Gebhard F, Weidner A, Liener UC, Stöckle U, Arand M. Navigation at the spine. Injury. 2004; 35: S-A35-45.
- Tjardes T, Shafizadeh S, Rixen D, Paffrath T, Bouillon B, Steinhausen ES, et al. Image-guided spine surgery: state of the art and future directions. Eur Spine J. 2010; 19: 25-45.
- Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009; 64: 231-239.
- Zausinger S, Scheder B, Uhl E, Heigl T, Morhard D, Tonn JC. Intraoperative computed tomography with integrated navigation system in spinal stabilizations. Spine (Phila Pa 1976). 2009; 34: 2919-2926.