
Case Report
Austin Neurosurg Open Access. 2016; 3(2): 1049.
Possible Self-Healing of Cerebral Cavernomas
Pozzati E*, Valluzzi A and Fioravanti A
Department of Neurosurgery, Bellaria Hospital, Bologna, Italy
*Corresponding author: Eugenio Pozzati, Department of Neurosurgery, Bellaria Hospital, Bologna, Italy
Received: July 27, 2016; Accepted: August 22, 2016; Published: August 24, 2016
Abstract
The natural history of cerebral cavernous malformations, or cavernomas, is generally discussed in terms of progression including in particular the hemorrhagic course which constitutes the most important risk of the disease. Otherwise, long lasting regression and quiescence may occur, but the chance of a non-surgical cure has been never considered.
Cerebral cavernomas have been divided in four types on the basis of their histopathological features and Magnetic Resonance Imaging. Type I generally corresponds to a subacute hemorrhage surrounded by a ring of hemosiderin and gliotic parenchyma. We describe one case of durable clinical and radiological self-healing of a sporadic Type I cavernoma consisting of a residual ring of hemosiderin staining close to a small Developmental Venous Anomaly (DVA).
We believe that some cavernous malformation may vanish after hemorrhage through a short-circuit in their development at a very immature stage. The impact of a possible self-cure on the management of this malformation should be better evaluated in the future.
Keywords: Cavernous malformation; Development venous anomaly; Epilepsy; Healing; Hemorrhage; Magnetic resonance imaging
Introduction
Cerebral Cavernomas or Cavernous Malformations (CMs) are hemorrhagic lesions consisting of clusters of endothelial sinusoidal channels, filled with blood in various stages of organization, intermingled with dense connective matrix and surrounded by hemosiderin stained gliotic parenchyma. These histopathological features configure a mature lesion and correlate with the four types of CMs classified on the basis of their Magnetic Resonance Imaging (MRI) findings [1]. They exist in two forms, familial and sporadic with different pathophysiology: most importantly, the sporadic form is very often associated to a Development Venous Anomaly (DVA). CMs are dynamic lesions growing and shrinking, rarely remaining quiescent and never self healing [2,3]. They may present with seizures, hemorrhage and focal deficits. In particular, the course of hemorrhage is unpredictable and the major risk is its recurrence: for this reason, surgical treatment is often considered. We report an apparent selfcure of an hemorrhagic cavernoma that arrested at Type I lesion and involuted leaving only its ring-shaped hemosiderin staining without residual malformation.
Case Report
In January 2010, this 40 year-old healthy man presented with a focal motor seizure involving the right arm. Computed tomography scan on admission showed a hypodense, roundish and well demarcated lesion in the left parietal region. T1 and T2 Weighted Images (WI) showed a subacute focus of hemorrhage surrounded by a hypointense rim of hemosiderin stained brain and cerebral swelling, consistent with a cavernous malformation. After contrast medium administration a small associated DVA was found (Figure 1). A surgical excision was planned, but the patient preferred to postpone the operation due to familial problems. In August 2010, MRI was repeated, but the lesion had disappeared leaving only a residual ring shaped hemosiderin deposition close to the DVA on Gradient Recalled Echo (GRE) sequences (Figure 2). The operation was not performed. Three years later the patient had a partial motor seizure: follow-up MRI confirmed the previous favourable findings and the electroencephalogram was normal. When seen in 2015, he remains well without antiepileptic medication.
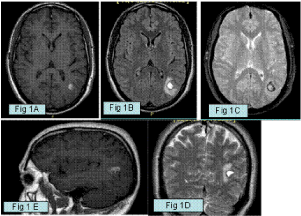
Figure 1: A,B,C: Axial T1 and T2 weighted images (WI), Flair and Turbo Spin
Echo (TSE) Magnetic Resonance Imaging (MRI) showing a subacute focus of
hemorrhage surrounded by a rim of hemosiderin stained brain consistent with
a Type I cavernous malformation in the left parietal lobe. D: Coronal T2 WI
showing loculation and fluid level of the hemorrhagic cavernous malformation.
E: Sagittal T1 WI after contrast medium administration showing a small deep
venous anomaly draining the cavernoma.
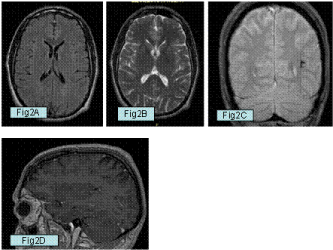
Figure 2: A,B: Axial T1 and T2 WI taken eight months later showing a
complete disappearance of the cavernoma. C: Coronal Gradient Recalled
Echo (GRE) sequence shows a ring-shaped hemosiderinic residual
corresponding to the vanished malformation. D: Sagittal T1 weighted image
after contrast medium administration showing the residual deep venous
anomaly without cevernoma.
Discussion
The natural history of cerebral cavernomas is generally considered in terms of risk factors and “progression”, including hemorrhagic course and growth of the lesion: otherwise, definitive “regression” indicating an anatomic involution and a long term favourable clinical course has been rarely discussed. The concept of “healing” of a CM is undoubtly hazardous and implies a durable and yet undetermined clinical follow-up and radiological imaging. As occurred in our case, and in spite of radiological resolution on T1 and T2 WI, some risk of epilepsy may persist due to the residual hemosiderin deposition which is the indelible marker of this malformation.
The imaging of CMs is time-dependent. Among the four types of CMs described on the basis of their MRI appearance, type I is a transitory form characterized by subacute hemorrhage hyperintense on T1 and T2 WI surrounded by hemosiderin stained gliotic brain tissue which appears as a black ring. It constitutes about 6% of CMs and a common presentation of de novo lesions. This CM often demonstrates a spontaneous decrease in size during follow-up due to blood absorption [2], but the matrix of the lesion becomes evident in the form of the classic “honeycombe” Type II lesion, which represents the mature and active form of the disease [1,2]. In some cases, the regression jumps to the indolent type III lesion consisting of a tiny hemosiderin staining around and within the thrombotic material inside the lesion, but even in this more favourable subset of patients, some growth or hemorrhage may later occur [4]. Type IV lesions can only be seen in GRE sequences as hypointense foci: they are tiny CMs or capillary telangiectasias and possibly represent true new lesions.
Our case may suggest that some Type I CMs at an immature stage may run an abortive course that can be confused with a resolving spontaneous hematoma. The final radiological appearance may consist of the evocative ring shaped hemosiderin deposition representing the inactive hemorrhagic scar, better if close to an associated DVA, which witnesses the malformative origin of the hemorrhage. DVAs are non hemorrhagic and almost asymptomatic malformations and associated bleeding is generally attributed to a combined CM.
The understanding of a self-healing [5] needs a great insight into the origin and pathophysiology of CMs.
Experimental studies have demonstrated that in the early stage CMs resemble intact endothelial “bubbles” and are inducted in a restricted time frame [6]. The existence of a “noncollagenized” CM representing a primitive form has been also suggested in other experimental models [7] and described as “en dentelles” configuration. A switch in the endothelial to mesenchymal phenotype has been described in CMs, which may represent the key molecular transition from immature to mature malformations [8]. The MRI appearance of a CM at this stage may be problematic. In a MRI taken few days before the appearance of a de novo hemorrhagic CM [5], we had found a roundish decreased signal intensity consistent with deoxyhaemoglobin at the site of the native CM, supporting the concept of endothelial chambers with stagnant circulation in the early stage. In a previous paper on mixed CMs of the brainstem [9], we had described a purely endothelial malformation, presumably representing a transition toward a mature form. Some melting and fading of these endothelial spaces with an associated DVA was also observed, better explaining the pathological substrate of the interface CM/DVA and the possible switch of venous endothelial cells to form CMs at the level of the terminal venous radicles [6].
Cavernomas are not visualized with angiography and have been included in the past in the so called Angiographically Occult Vascular Malformations (AOVMs): MRI apparently covers the full pathological spectrum of CMs, but some “gap” of imaging may exist also for this technology. Although CMs have been generally considered congenital, it is known that they may be acquired and appear truly de novo in normal areas of the brain, in particular after irradiation or close to DVAs [4]: alternatively, some pre-existent form of MRI “occult” cavernoma may debut with an early hemorrhagic short-circuit disrupting the pristine endothelial spaces [3,10]. The field of true “de novo” CMs becomes progressively smaller as ultrahigh field MRI at 7T or over is used and reveals very immature lesions [11]. The present case should reinforce the current search for pharmacological inhibition and involution of CMs within the short therapeutic window at this stage.
The mechanism favoring the healing of CMs is unclear: the self-destruction of the vascular malformation with bleeding is an intuitive occurrence which may leave inconsistent endothelial debris [3]. The spectrum of the sporadic form probably encompasses some cavernomas at low penetrance: their pathogenesis and evolution should be better investigated through the genetic folds of the “two hit” molecular mechanism of the disease.
Conclusion
A five year follow-up without radiological recurrence may suggest a substantial healing of the type I in our case: the impact of a self-cure on the management of cerebral cavernomas should be better evaluated in the future.
References
- Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994; 80: 422-432.
- Clatterbuck RE, Moriarity Jl, Elmaci I, Lee RR, Breiter SN, Rigamonti D. Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg. 2000; 93: 981-986.
- Pozzati E, Acciarri N, Tognetti F, Giangaspero F. Growth, subsequent bleeding and de novo appearance of cavernous angiomas. Neurosurgery. 1996; 38: 662-670.
- Pozzati E. Thalamic cavernous malformations. Surg Neurol. 2000; 53: 30-40.
- Pozzati E. Cavernous malformations as dynamic lesions: de novo formation, radiologic changes and radiation induced forms. Lanzino G, R Spetzler, editors. In: Cavernous Malformation of the brain and spinal cord. Thieme Ed. 2007; 30-40.
- Boulday G, Rudini N, Maddaluno L, Blecon A, Arnould M, Gaudric A, et al. Developmental time of CCM2 loss influences cerebral cavernous malformations in mice. J Exp Med. 2011; 208: 1835-1847.
- Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 sensitizes mice with mutation in Ccm 1 (KRIT1) to development of cerebral vascular malformations. Am J Pathol 2004; 165: 1509-1518.
- Bravi L, Malinverno M, Pisati F, Rudini M, Cuttano R, Pallini R, et al. Endothelial cells lining sporadic cerebral cavernous malformations undergo endothelial to mesenchymal transition. Stroke. 2016; 47: 886-890.
- Pozzati E, Marliani AF, Zucchelli M, Foschini MP, Dall’Olio M, Lanzino G. The neurovascular triad: mixed cavernous,capillary and venous malformations of the brainstem. J Neurosurg. 2007; 107: 1113-1119.
- Pozzati E, Giangaspero F, Marliani F, Acciarri N. Occult cerebrovascular malformations after irradiation. Neurosurgery. 1996; 39: 677-682.
- Dammann P, Barth M, Zhu Y, Maderwald S, Schlamann M, Ladd M, et al. Susceptibility weighted magnetic resonance imaging of cerebral cavernous malformations: prospects, drawbacks and first experience at ultra-high field strength (7-Tesla) magnetic resonance imaging. Neurosurgical Focus. 2010; 29: 1-7.