
Case Report
Austin Neurosurg Open Access. 2016; 3(2): 1052.
Delayed Treatment of Large Scalp and Skull Defects Resulted from Trauma: A Case Report
Yang C*, Wu C and You C
Department of Neurosurgery, West China Hospital of Sichuan University, China
*Corresponding author: Chaohua Yang, Department of Neurosurgery, West China Hospital of Sichuan University, No.37 Guoxue Road, Chengdu, China
Received: August 29, 2016; Accepted: September 23, 2016; Published: September 26, 2016
Abstract
Open craniocerebral injury should be treated as soon as possible, especially for those patients with scalp defects and brain tissue exposure. If debridement cannot be performed in time, infection will be more likely to occur. Here, we reported the treatment of a patient with large scalp and skull defects who got treatment 24 h post-injury and developed severe infection and presented our experience.
Keywords: Open craniocerebral injury; Infection; Skin transplantation
Introduction
Debridement can remove potential contaminants and aid in wound closure for patients with an open craniocerebral injury. Surgical treatment should be performed within 12 h of the injury to decrease the risk of infectious complications [1,2]. Delayed treatment is of great troublesome because of infection. In the present case report, we described the delayed treatment of a patient with large scalp and skull defect.
Case Presentation
A 5-year-old girl was admitted to our hospital for coma 3 days after a traffic accident. Three days before admission the girl was hit by a car when she was walking on the roadside, which leading to coma and defects of scalp and skull in left frontal, temporal and parietal region. Post-injury 24 h, the girl reached local hospital and debridement was performed. Later, the patient was transferred to our hospital for coma and fever.
Upon admission, the patient was in coma and with endotracheal intubation. The patient had a Glasgow Coma Scale score of 8; her pupils were uniformly round, exhibited isocoria, and were found to be sensitive to light. Missing scalp tissue and bones over the left frontal, temporal, and parietal areas were noted, and brain tissue could be seen (Figure 1). Purulent discharge and contaminants were found at the wound sites. Head computed tomography revealed encephalocele, and that portions of the left frontal, temporal, and parietal bones were missing (Figure 2).

Figure 1: On admission, scaple, skull, and dura defect could be found in left
frontal, temporal, and parietal areas. Purulent discharge and necrosis tissues
were found at the same time.
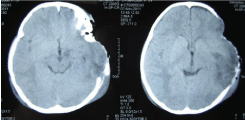
Figure 2: Computed tomography showed left frontal, temporal bone defect
and encephalocele. Foreign object was found in left temporal.
Tracheotomy and debridement were performed immediately after admission. Scalp tissue and bones over the left frontal, temporal, and parietal areas were defective. Moreover, a portion of the dura was also found to be missing. Hydrogen peroxide and saline were used to rinse the wounds and remove foreign object and contaminants such as hair and dust; necrotic brain tissue was also removed, and the wounds were covered with Vaseline gauze and saline-soaked gauze. Antibiotics were administered following the operation. Another debridement was performed 2 days later; necrotic tissue, pus collection, and necrotic dura were removed. After the two operations, the patient’s temperature fluctuated between 34°C and 40°C. The secretions from the infected wounds were cultured several times to identify the causative pathogens. Flavobacterium odoratum, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii–Acinetobacter calcoaceticus complex isolates were found in those tests. Sputum culture revealed that the main pathogens were Acinetobacter baumannii, Acinetobacter haemolyticus, and Pseudomonas aeruginosa. Tazocin, vancomycin, cefoperazone/ tazobactam, and tienam were consecutively used for antibiotic treatment. After treatment with a combination of debridement and antibiotics and wound dressing change, the patient regained consciousness, and her temperature returned to normal on the 39th day after admission. The patient exhibited grade I muscular strength with normal muscular tension in the right limb, and grade V muscular strength, also with normal muscular tension, in the left limb. As infection in the wound was completely controlled and granulation tissue was fresh (Figure 3), skin transplantation was performed on the 51st day after admission. The defect areas were covered by a rotating scalp flap from the right frontal and parietal areas. Subsequently, free skin flaps were taken from the left side of the back and transplanted to cover the donation site on the right frontal and parietal areas. The transplanted skin flap had sufficient blood supply and acquired a ruddy color. After operation, the transplanted skin survived (Figure 4). The patient recovered and was discharged on the 71st day after admission. Six month after injury, the patient’s Glasgow Outcome Score was 4 (Figure 5). However, the skull defects are still waiting for treatment.

Figure 3: Infection was completely controlled and granulation tissues were
fresh.
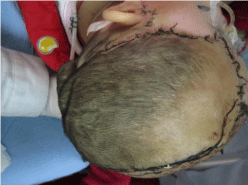
Figure 4: Rotating scalp flap was survived after transplantation.

Figure 5: Six months after operation, skin transplantation was successful.
Discussion
At present, many patients with traumatic brain injury can be treated within the so-called “golden hour”. It’s very important for patients’ outcomes. Especially for open craniocerebral injury, debridement should be performed to remove foreign object and contaminants and make wounds closure as soon as possible. However, some patients with open craniocerebral injury cannot be debrided in time and may suffer infection. If infection is happened, the treatment will be more difficult. As the patient in our report, she lived in a remote mountain area and could not reach hospital in time. The first debridement was performed 24 h post injury. Moreover, there was still some foreign object remained. All these resulted in severe infection.
In the present case, the patient was transferred to our hospital 3 days after the injury. It’s very important to perform a total debridement to remove the foreign object, necrotic tissue and pus collection. Debridement can be repeated when it is needed. At the same time, antibiotics therapy is also very important. At the beginning, the use of antibiotics was in an experience-based manner. Later, antibiotics were adjusted based on the results of drug sensitivity tests and the patient’s treatment reaction. Staphylococcus aureus is the most frequently associated organism. However, gram negative bacteria also frequently cause intracranial infection after penetrating brain injury [3]. Several kinds of bacteria’s were identified in the case. It is recommended that broad spectrum antibiotics should be administered in all penetrating brain injury cases, and must be started as soon as possible [4].
Skin grafting is performed only after infection was controlled and granulation tissues were fresh. As the scalp, skull and dura were all defective and cerebral spine fluid leakage was found, whole skin transplantation was considered to cover the wound and prevent cerebrospinal fluid leakage. Unfortunately, left superficial temporal artery was also injured, and skin flap with vascular anastomosis could not be performed. Therefore, rotating flap from the right frontal, temporal, and parietal was made and transplanted to the wound site, and free skin grafting was performed on the donor site. It is important to perform subdural drainage until the wound is healing and no further cerebrospinal fluid leakage is found. At last, the patient got well. However, additional cranioplasty will be performed to repair the remaining skull defects. In conclusion, debridement, control infection, and skin transplantation are the key points to the delayed treatment of a patient with large scalp and skull defects.
References
- Helling TS, McNabney WK, Whittaker CK, Schultz CC, Watkins M. The role of early surgical intervention in civilian gunshot wounds to the head. J Trauma. 1992; 32: 398-400.
- Hubschmann O, Shapiro K, Baden M, Shulman K. Craniocerebral gunshot injuries in civilian practice: Prognostic criteria and surgical management experience with 82 cases. J Trauma. 1979; 19: 6-12.
- Bayston R, de Louvois J, Brown EM, Johnston RA, Lees P, Pople IK. Use of antibiotics in penetrating craniocerebral injuries. “Infection in Neurosurgery” Working Party of British Society for Antimicrobial Chemotherapy. Lancet. 2000; 355: 1813-1817.
- Kazim SF, Shamim MS, Tahir MZ, Enam SA, Waheed S. Management of penetrating brain injury. J Emerg Trauma Shock. 2011; 4: 395-402.