Research Article
Austin J Nucl Med Radiother. 2014;1(1): 3.
Dose Calculation for I131/ I124 Radioimmunoconjugates for Radioimmunotherapy
Bloch P and Plastaras JP*
Department Radiation Oncology, University of Pennsylvania, USA
*Corresponding author: Plastaras JP, Department of Radiation Oncology, Perleman Center for Advanced Medicine, University of Pennsylvania, 3400 Civic Center Boulevard, Philadelphia, PA 19104, USA
Received: August 20, 2014; Accepted: September 19, 2014; Published: September 24, 2014
Abstract
For clinical evaluation of Radioimmunotherapy (RIT) it would be valuable to measure the heterogeneity of the uptake and retention within the tumor bed of the radioimmunoconjugate agent. Presently, the patient-specific maximally tolerated therapeutic amount of radioactivity to administer is determined from measurements, prior to treatment, of the whole-body clearance of a trace amount of I-131 labeled radioimmunoconjugate agent. Labeling the therapeutic radioimmunoconjugate agent with the positron emitter I-124 would permit PET imaging to be employed to determine the regional uptake and retention of the therapeutic administered agent. An algorithm has been developed to calculate the 3D-dose distribution of I-124 and I-131 using Monte Carlo derived point dose kernels and measured regional Specific Uptake Values (SUVs) and clearance determined from PET/CT studies. The calculations indicate that for the same concentration of activity in a tumor volume (uCi/gm), I-124 would result in a higher dose rate (Gy/hr) and greater total dose than I-131.
Keywords: Cancer therapy; Radioimmuniotherapy; Dosimetry iodine-124/131
Introduction
To evaluate the dose delivered to the tumor and normal tissues for Radio Immunotherapy (RIT) requires measured data of both the regional distribution and the pharmacokinetics of the radio labeled agent. Therapeutic amounts of I-131 have been administered for RIT [1]. Although estimates of the dose deposition have been attempted using SPECT, detailed spatial information on the distribution of the I-131from nuclear medicine studies is limited by the physical properties of the isotope [2]. Attaching a positron emitter such as I-124 to the radioimmunoconjugate would permit using PET/CT imaging to determine the spatial distribution of the isotope in both the tumor and normal tissues.
In this paper we evaluate and compare the dosimetric differences of I-124 and I-131. For dosimetry proposes the decay products of isotopes are usually separated into two components; (1) a beta like component where the energy emitted is absorbed at the point of emission and (2) a gamma like component where the energy emitted is absorbed at a distance from them point of emission. ENSDF* data were used to evaluate these two components for I-124 and I-131. The equilibrium energy emission constant for the beta like Δβ and gamma components Δγ shown below demonstrates two important features:
Iodine 124 (g-rad/uCi-hr)
Iodine 131(g-rad/uCi-hr)
Beta Δβ
0.414
0.408
Gamma Δγ
2.347
0.815
Total Δβ + Δγ
2.753
1.222
features:
(1) The beta and Auger electron component of the dose are similar for both isotopes of iodine and (2) the gamma component of the dose is significantly greater for Iodine-124. Thus one would expect for the same concentration of activity (uCi/g) a higher dose rate would be obtained using I-124 for RIT. More detailed calculation of the regional dose distribution obtained during RIT requires both information on the regional uptake of the radioimmunoconjugate and a point dose kernel for the two iodine isotopes.
* Evaluated Nuclear Structure Data File (ENSDF) in the Medical Internal Radiation Dose (MIRD) Format.
Method
An algorithm written in Interactive Data Language (IDL) has been developed for evaluating the 3D dose distribution for iodine labeled radio-immunotherapy (RIT) for the two isotopes of Iodine 124 & 131. The algorithm to calculate the 3D regional dose distribution can use the regional distribution of the uptake of the positron emitter I-124 obtained from PET/CT studies.
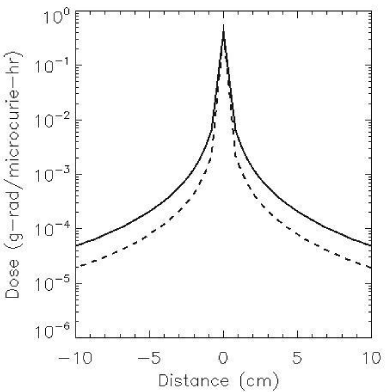
Papadimitroulas et.al [3] used Monte-Carlo simulations to calculate the dose distribution in water for point sources of I-124 and I-131. Using these data a point dose- kernel was calculated on a voxel grid of 4 mm ∧3, corresponding to the voxel size used to acquire PET images. Figure 1 shows the point dose kernel on a 4 mm grid for the two isotopes of Iodine. The range of the beta particles for both isotopes is approximately 4 mm. It was therefore assumed in generating figure 1 that the energy absorbed at the voxel containing the point source is the equilibrium energy emission constant of the beta like component Δβ for each isotope. The Monte-Carlo calculations fail to constrain the energy emitted thus resulting in infinitely large doses near the source.
Figure 1 : Point Dose Kernel on 4 mm grid. I-124 (solid line) I-131 (dash line).
Results
The point dose kernel (Figure 1) demonstrates that the beta dose rate near the source is similar for both isotopes, whereas the dose rate is greater at larger distances forI-124 than I-131, due to the larger gamma component of I-124. The algorithm for calculating the 3D dose distribution convolutes the point-dose kernel with the calibrated Standardized Uptake Values (SUVs) obtained from the PET images.
Comparison of I-124 and I-131 dose rate distributions
Convolutions were performed over a 31 x 31 x 31 matrix (124mm∧3) taking approximately 100 seconds on a MAC PRO1.1 computer to complete a 3D dose calculation for a phantom of 200 ^3 voxels or a volume of 800^3 mm. There was no significant difference in the calculated dose using a smaller convolution volume i.e. 15x 15 x15 voxels.
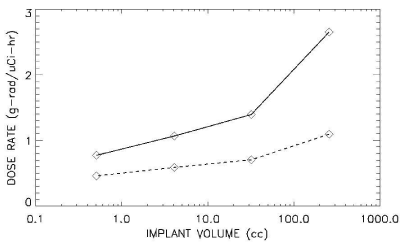
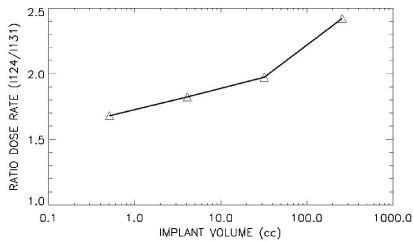
Dose distribution and dose volume histograms were calculated for uniform distributions for both isotopes of iodine. The dose rate within different simulated target volumes containing the same concentration of activity was calculated for both iodine isotopes. Figure 2 (top) shows the calculated dose rate delivered to 90% of each of the simulated target volumes. The dose rate is greater for I-124 by a factor of between 1.6 and 2.4 (Figure 2 bottom) depending on the simulated volume containing the uniform concentration of activity.
Figure 2: (Top): Dose rate as a function of implant volume for I-124 (solid) and I-131 (dash) lines.
Figure 2:(Bottom): Ratio of the (I-124/I-131) dose rates with implant volume.
In the clinical use of RIT, the uptake will likely not be uniform. For clinical considerations the isotope that produces a dose distribution that is less sensitive to small regional changes in uptake would be more desirable.
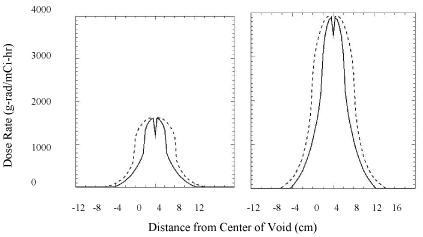
To test the influence of uptake heterogeneity on the dose distribution a 12x12x4 mm∧3 simulated void containing no iodine was placed in the center of an 80 x 80 x 40 mm∧3 iodine containing target volume. Figure 3 shows a perturbation in the dose of approximately 30% with I-131 and 13% with I-124. The larger gamma dose component associated with I-124 helps to reduce dose heterogeneity resulting from small changes in the regional uptake of the isotope.
Figure 3 : Perturbation of dose-rate due to a small 12 x 12 x 8 mm void (zero iodine concentration) at the center of a solution of a radioactive iodine I-131 (left) or I-124 (right) having a volume 80 x 80 x 40 mm∧3. Dose profiles through the center along both the long (dashed) and short (solid) axes of phantom.
Total Dose Distributions for I-131 & I-124:
The total dose during RIT depends on the cumulative activity, which depends on the physical and biological uptake and clearance of the RIT agent. The cumulative activity A (cum) for the special case of an instantaneous uptake A(0) and a constant biological clearance rate is given by the following expression
A(cum)=A(0)τεφφ/0.693
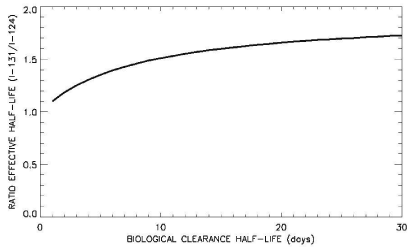
where 1/ τεφφ = 1/τb + 1/ τp and sb, τp are the biological and physical half-lives respectively. For no biological clearance of the isotopes, the ratio of the effective half-life of I-131/I-124 is the ratio in the half-life of the two isotopes (8.02 days/4.176 days) =1.92. Thus with no biological clearance of the agent, the data presented in Figure 2 (bottom) indicates that for simulated target volumes greater than approximately 10cc the total dose delivered would be greater for the I-124 labeled agent. However with a finite biological clearance half-life of less than approximately 230 days the ratio of τεφφ shown in Figure 4 is substantially reduced resulting in a greater total dose from I-124 for even the smaller simulated target volumes shown in Figure 2.
Figure 4 : Ratio of τeff for I-131/I-124 as a function of biological clearance half-life.
Summary and Conclusion
Monte-Carlo derived point dose kernels were used in an algorithm, written in IDL, to calculate the 3D dose rate distribution and Dose Rate Volume Histogram (DVH) for volumes containing either I-124 or I-131 labeled radioimmunoconjugates. The calculated dose distributions were significantly different for the two isotopes of iodine. The data demonstrates that for the same concentration of activity I-124 would result in a greater dose-rate within the treatment volume. In addition, the calculated dose rate distribution within the target volume indicates that I-124 would be less sensitive to heterogeneity in the regional uptake of the RIT agent.
As interest in I-124 increases with multiple options for production, the I-124 isotope will hopefully become more widely available [4]. At present, I-124 is much more expensive than I-131, however its dual properties of being imaged with PET while being able to deliver radioimmunotherapeutic dose makes its development intriguing. In addition to conjugating to antibodies, alternate localizing molecules can be used. One such I-131/I-124 dual agent is being developed for based on the relative overabundance of phospholipid ethers in cancer cells [5]. Labeling the radioimmunoconjugate with the positron-emitting isotope I-124 permits using PET/CT studies to measure the regional distribution and pharmacokinetics of the therapeutic agent within both the tumor and normal tissues in patients undergoing a course of RIT. Knowledge of the regional uptake of the RIT agent allows one to evaluate the dose-rate heterogeneity within the tumor-bed. In addition, serial PET studies could be obtained with the I-124 labeled radioimmunoconjugate to assess regional temporal changes in the SUVs associated with the biological clearance of the agent, which is necessary for evaluating the total dose, and dose-volume delivered during the course of RIT.
References
- Wahl RL. The clinical importance of dosimetry in radioimmunotherapy with tositumomab and iodine I 131 tositumomab. Semin Oncol. 2003; 30: 31-38.
- Dewaraja YK, Schipper MJ, Roberson PL, Wilderman SJ, Amro H, Regan DD, et al. 131I-tositumomab radioimmunotherapy: initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med. 2010; 51: 1155-1162.
- Papadimitroulas P, Loudos G, Nikiforidis G, Kagadis G. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: Comparison with other Monte Carlo codes. Medical Physics. 2012; 39: 5238-5247.
- Braghirolli AM, Waissmann W, da Silva JB, dos Santos GR. Production of iodine-124 and its applications in nuclear medicine. Appl Radiat Isot. 2014; 90: 138-148.
- Weichert JP, Clark PA, Kandela IK, Vaccaro AM, Clarke W, Longino MA, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med. 2014; 6: 240ra75.