Research Article
Austin J Nucl Med Radiother. 2014;1(1): 5.
Estimated Clinical Impact of Fractionation Scheme and Tracking Method upon Imaging Dose in Cyberknife Robotic Radiosurgery
Christopher J Tien1, Sung-Woo Lee*2, Sonja Dieterich1
1Department of Radiation Oncology, Community Hospital, USA
2Department of Radiation Oncology, Rhode Island Hospital / Brown University, USA
3Department of Radiation Oncology, University of California, USA
*Corresponding author: Sung-Woo Lee, Department of Radiation Oncology, Rhode Island Hospital / Brown University, USA
Received: September 10, 2014; Accepted: October 03, 2014; Published: October 06, 2014
Abstract
Image guidance provides significant gains in targeting accuracy - which leads to smaller planning margins and higher dose conformity - leading to smaller overall doses. This suggests that there may be a balance between increased imaging dose in therapeutic applications and the ALARA philosophy stressed by the physics community. In order to optimize the imaging dose which is delivered, it is essential to estimate and understand important parameters which govern imaging dose. In the case of CyberKnife (CK) imaging dose, two significant factors were fractionation and tracking method. These choices are dictated by lesion treated. Therefore, a cohort of 427 patients from two CK institutions treating very different patient populations was retrospectively studied. The number of images had taken varied 1200% between one-fraction skull tracking and three-fraction spine tracking - with 50% of patients receiving estimated entrance skin exposure of 0.8 Gy. Other tracking methods, such as fiducial tracking and lung tracking produced a median estimated entrance skin exposure of 1.5 and 3.0 Gy, respectively. Results from both institutions clearly demonstrated the effects of fractionation scheme and tracking method upon the imaging dose in CK SRS can be substantial if not carefully managed.
Keywords: Image-guided radiotherapy; Stereotactic radiosurgery; Robotic radiosurgery; Radiation therapy
Possible PACS Numbers
87.53.Jw (therapeutic applications); 87.53.Ly (stereotactic radiosurgery); 87.56.Da (ancillary equipment); 87.53.Bn (dosimetry of ionizing radiation); 87.57.uq (medical imaging dosimetry)
Introduction
Image guidance has become standard practice for all types of radiation therapy because it imparts increased target accuracy leading to smaller planning margins and higher dose conformity [1]. The total dose for image-guided radiation therapy (IGRT) is the sum of the prescription therapeutic dose and the dose from imaging itself [1]. However, due to smaller imaging dose amount compared with total prescription dose could make importance of limiting or optimizing imaging dose under-estimated. Imaging dose includes computed tomography (CT) scan for planning as well as both interfraction and intrafraction imaging [1]. Depending on the institution and treatment protocol, typical imaging can be anywhere from two interfractional conventional kilovoltage (kV) radiographic images to daily cone-beam CT (CBCT) for conventional linacs. In addition, the time span can be spread over a few weeks, as in conventional radiotherapy, or condensed into two hours or less, as in radiosurgery.
Imaging dose has been discussed by the American Association of Physicists in Medicine (AAPM) Task Group (TG) 75, which concluded that imaging dose can be reduced to relatively negligible levels for most radiotherapy applications discussed [1]. However, this report only briefly addressed robotic radiosurgery [1]. Along with radiosurgery linacs such as BrainLab's ExacTrac system, CBCT and portal and fluoroscopic imaging does were referred, but site specific dose estimation per image was only mentioned. Later, AAPM TG 135 focused on robotic radiosurgery, but referred imaging dose estimates back to AAPM TG 75 [2]. Neither the estimated number of images nor distribution of images were reported by the task group reports, which precluded an estimate of imaging dose [1,2].
In therapeutic applications, regardless of whether imaging dose is employed, there is already an extremely high level of radiation dose. Using image guidance provides significant gains in targeting accuracy, which may lead to smaller planning margins and reduced dose to normal structures. This leads to a trade-off between increased imaging frequencies in order to increase targeting accuracy vs. reduced imaging in order to lower imaging dose. To optimize the imaging dose which is delivered, it is essential to understand important parameters which govern imaging dose.
In the case of CyberKnife (CK) imaging dose, the two possible contributors which were investigated in this study were fractionation and tracking method. Studies by Hoogeman et al. and Xie et al. provide general insight into the extent of imaging in CK lung and prostate procedures, respectively [3-5]. However, the effects of both fractionation and tracking method has not been studied in literature.
This investigation retrospectively estimated the extent of imaging done for 447 cases from two different CK institutions which have very different patient populations - which resulted in very different fractionation and tracking method choices. An estimate of imaging dose is provided as well as an explanation discussing the impact of fractionation and tracking method.
Materials and Methods
Institutions and treatment data
Information on imaging frequency, total number of images, exposure factors (kV, mA and exposure time), fractionation schemes, and treatment site has been collected retrospectively on 427 radiosurgery and stereotactic body radiation therapy (SBRT) patients treated in the previous two years at two institutions. These particular institutions were chosen because the intra-institutional patient demographics, treatment site, and prescription were very different. This facilitated assembly of a diverse cohort of patients and treatment patterns.
Dose conversion
This study uses entrance skin exposure (ESE) as suggested in TG75 for kV imaging as an alternative for effective dose, which is particularly convenient in radiotherapy because of skin injury concerns [1]. Forty five exposure measurements were made per tube using a diagnostic-class ionization chamber to determine the ESE per image. The preliminary exposure measurements were performed with a 6 cc RadCal ion chamber at the iso-center, which distance was 230 cm from the focal spot of each x-ray tube. Exposure measurements were made over ranges of 80 kV to 150 kV, 50 mA to 320 mA, and 50 msec to 640 msec. The total number of x-ray images associated with each CK treatment was retrieved. Then, the ESE from imaging was estimated to the different anatomic treatment regions, such as brain, thorax, spine, and abdomen/pelvis, using the exposure measurements.
Accuray cyberKnife® G-4 model
The Accuray CK consists of a linear accelerator mounted on an industrial robotic manipulator arm which aims the linear accelerators collimated beam at a target region with sub-millimeter targeting accuracy [6]. The imaging system consists of two kV x-ray tubes and two accompanying floor-mounted amorphous silicon flat-panel detectors with an active area of 25 cm2. The x-ray tubes have 2.5 mm Al filtration and are typically operated at 120 kV, 100 mA and 100 ms exposure time. Each tube is capable of 40-125 kV, 25-320 mA, and 1-640 ms. The x-ray tubes use fixed collimators and are ceiling-mounted at a 45 degree angle with respect to the plane of the floor on opposite sides of the treatment table to provide orthogonal views.
The imaging system's set-up differs from conventional radiography in three ways. First, the system has a fixed field of view because it does not use an adjustable collimator. Secondly, the source-to-detector distance (SDD) is 330 cm, which is more than three times longer than the typical 100 cm SDD radiography set-up. Finally, the detectors are generally mounted flush with the ground. Therefore, the x-rays are not directly incident upon an image receptor, but instead impinge at a 45-degree angle, which reduces image quality due to geometric distortion.
CyberKnife treatment delivery
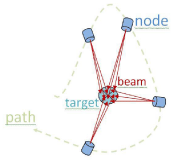
During a CK treatment procedure, the linear accelerator moves around the patient along a predetermined path and dwells at different predetermined locations in space called nodes, where beams of varying intensity are delivered to the target. The robotic arm can achieve up to 120 different source positions known as nodes, and can be manipulated up to 10 different angles at each node. This allows us to have up to 1200 different beam positions. Typically, a procedure will last around 15-60 minutes, with 30-50 nodes and about 40-120 beams. The concept of nodes, beams, targets, and path is illustrated in Figure 1 below. The general path of machine is calculated prior to delivery, but is adjusted in real-time using the image-guided tracking methods will be described in the next section.
Figure 1 : CyberKnife's beam, node, path, and target illustrated.
CyberKnife® image-guidance tracking methods
There are four major image-guidance (tracking) methods currently available in the CK system: skull tracking, spine tracking, lung tracking, and fiducial tracking. In all methods, real-time x-ray imaging is matched to digitally reconstructed radiographs generated by the planning system using the 2D-3D tracking algorithm [7]. The current version of CK software takes images based upon time intervals ("time-based") - n seconds per image, with n determined by the user. Previous versions of CK software were based upon number of beams delivered ("beam-based") - n beams delivered per image, again with n determined by the user.
Skull tracking follows bony landmarks within the cranium while spine tracking follows the spinal anatomy for lesions located in the spine or lung lesion proximal to spine tracking volume [8-11]. Lastly, fiducial tracking follows surgical markers as surrogates for the tumor motion [5,8-9]. The choice of tracking method is dependent upon the location of the target. Targets in the cranium are tracked using skull tracking. All spinal and paraspinal tumors that minimally move with respiration are tracked using spine tracking. Soft-tissue tumors not immediately abutting the cranium or spinal column must be used with fiducial tracking and Synchrony technique. The Synchrony technique is a method that correlates external infrared markers and internal fiducial movement and then predicts patient movement. According to this model, linac head tracks the tumor movement during the treatment. When abrupt patient movement occurred, this Synchrony model should be establish again to correlate external and internal patient movement.
Results
ESE measurement results
The average ESE for the brain region treatment was 17 cGy (3 - 53 cGy); for the thorax region treatment, 53 cGy (23 - 112 cGy); for the abdomen and pelvis region treatment, 41 cGy (17 - 111 cGy); and for the spine region treatment, 28 cGy (6 - 68 cGy). The estimated average additional ESE from imaging dose delivered to the patients was 1.2% of the prescription treatment dose (range 0.2% to 4.6%).
Institutional data
A summary of the distribution sorted by fractionation and tracking methods is shown in Table 1. Note that both fractionation and tracking method are dictated by the lesion treated. Thus, Table 1 mainly reflects the variation in patient populations between the two institutions. For example, Table 1 shows that Institution A delivered predominantly uses single fractionation while the predominant fractionation regimen at Institution B is three fractions. Table 1 also shows that Institution B did not treat pediatric cases, a small percentage of cranial treatments were pediatric cases at Institution A.
Institution A (n=220)
Institution B (n=207)
# Fx
Skull tracking
Spine tracking
Skull tracking
Spine tracking
Fiducial tracking
1
129
19
31
4
1
2
6
4
3
1
0
3
45
7
67
42
15
4
0
0
2
9
5
5
9
1
7
16
4
Table 1: Distribution of fractionation scheme and tracking methods for the two institutions studied.
Number of images taken
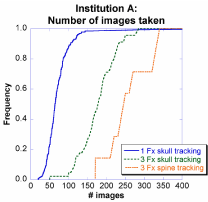
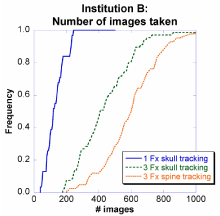
Figures 2 and 3 show the number of images taken at Institutions A and B as a cumulative distribution function (CDF) for the largest populated cases from Table 1: 1-fraction skull tracking, 3- fraction skull tracking, and 3-fraction spine tracking. Three features were observed in both institutions.
Figure 2: Distribution of number of images taken at Institution A for CyberKnife treatments.
Figure 3: Distribution of number of images taken at Institution B for CyberKnife treatments.
First, when employing spine tracking, the patient is imaged more frequently than when using skull tracking, resulting in a 150% increase in the number of images taken for the median number of patients. Specifically, at Institution A, the number of images taken (median) was 175 and 250, for patients tracked using skull tracking and spine tracking, respectively. At Institution B, the images taken (median) was 400 and 600, for patients tracked using skull tracking and spine tracking, respectively. As with fractionation, the relative numbers increased by 150%, but there was a two-fold difference in absolute magnitude which was presumably the result of the imaging interval used at Institution A being half of Institution B's. Overall, using different fractionation schemes and tracking methods, the images taken (median) varied by 1200%.
Second, over the course of treatment, patients who were treated with three fractions received approximately 400% more images than patients who completed treatment in only one fraction. For example, at Institution A, the images taken (median) was 50 and 175 for one-fraction and three-fraction patients, respectively. Similarly, at Institution B the images taken (median) was 100 and 400, for one-fraction and three-fraction patients, respectively. This was presumably the result of the imaging interval used at Institution A being half of Institution B's. The fractionation effect is explained in greater detail in Section IV.B.
Third, a patient at Institution B will most likely have much more images than at Institution A. For example, 1% of patients at Institution A receive >350 images while 90% of patients at Institution B receive >350 images.
Institution A also adjusted a substantial number of imaging parameters - including number of beams between images and exposure factors (kV, mAs, exposure time) while Institution B did not adjust its imaging parameters from the manufacturer's default settings.
Dose conversion
Organ doses are difficult to quantify mainly due to varying patient-specific parameters including source-to-surface distance, equivalent depth, organ locations, and variable field of view. ESE is a standard measure in planar kV which usually serves as a reasonable alternative for effective dose [1]. ESE is particularly convenient in radiotherapy because deterministic skin reaction is maximal at the skin's surface [1]. The ESE was measured to be 1.231 mGy per tube per image using a diagnostic ion chamber (10X6-6, RadCal, Monrovia, CA) placed at the imaging isocenter which agrees with estimates of TG-75 which range from 0.25 to 2.00 mGy per image [1].
Using a first-order approximation of 1.231 mGy ESE per image, the ESE varied between 61.6 to 738.6 mGy by changing fractionation and tracking method. This investigation was limited to studying two tracking methods - skull tracking and spine tracking. Other tracking methods, namely fiducial tracking and lung tracking did not have a large cohort at either applying the same ESE-to-image approximation as before, the median ESE in fiducial tracking (n=25) was 1,477 mGy (1200 images). The median ESE in lung tracking (n=5) was 3,200 mGy (2600 images). Compared with skull tracking, these sites also likely possess a higher effective-dose-to-image factor due to their field-of-view encompassing many more radiosensitive organs. It is evident from the limited sample sizes for fiducial and lung tracking that these tracking methods might result in significantly increased imaging dose.
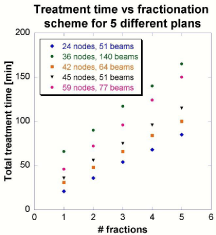
Total treatment time
The total treatment time is shown as a function of the number of fractions planned five patients mentioned earlier. The results are found in Figure 4 demonstrate a linear relationship between treatment time and fractionation scheme, with a coefficient of determination (R2) greater than 0.9989 for every plan.
Figure 4 : The effect of changing the number of fractions upon total treatment time.
The marginal time between fractions decreases as the number of fractions increases. For example, the patient plan with 24 nodes requires 46 minutes of treatment time in one fraction. However, in order to deliver the same amount of dose, only 72 minutes total are required for two fractions - 36 minutes per fraction. And, only 96 minutes total are required for three fractions, respectively - 32 minutes per fraction. The result is that 46, 36, and 32 minutes per session are required for one, two and three fractions, respectively. The treatment time normalized to number of fractions - along with the number of nodes and beams for the five patient plans displayed in Figure 4 - is shown in Table 2.
# Fx planned
Sample patient plan
A
B
C
D
E
1
21
66
31
36
46
2
18
45
24
28
36
3
18
39
22
25
32
4
17
35
21
24
31
5
17
33
20
23
30
nodes
24
36
42
45
59
beams
51
140
64
63
77
Table 2: Treatment time per fraction for five randomly selected patients sorted by the number of nodes in the treatment plan.
Discussion
Overall impact of fractionation choice and tracking method
This investigation was limited to studying two tracking methods - skull tracking and spine tracking. Other tracking methods, namely fiducial tracking and lung tracking, were excluded from in-depth analysis due to a small cohort size at both institutions. As a consequence of choosing a patient population across two separate institutions, the standard treatment approach - prescription dose, tracking method, and fractionation - was, in many cases, dramatically different. Therefore, the authors do not recommend a single universal reference standard. Rather, the authors strongly emphasize the potential heightened dose as a result of parameters as basic as fractionation or tracking method.
Specifically, tracking methods were shown to generate significant dose- with a median ESE up to 1.5 and 3.0 Gy, respectively. Choosing different fractionation schemes and tracking methods, the median entrance skin was up to twelve times higher. Other thing to consider for imaging dose for lesions in the T-spine region can be particularly detrimental due to the small effective path-length traversed due in the lung. This study did not consider a weighting factor to account for differences in tissue sensitivity: a high imaging dose is much more detrimental in lung tracking versus skull tracking.
Clinical impact of fractionation
It was observed that the total treatment time increased as the number of fractions increased while the time required for each individual treatment decreased. The total treatment time is the sum of beam-on time and machine travel time.
The total treatment time is important because the CK unit is time-based. The user selects a time interval for imaging - 30 seconds is the default for skull tracking cases - and this is the only factor. Switching from one-fraction to three-fraction treatments increased the median number of images taken by 350% and 400% for Institution A and Institution B, respectively. The reason why the ratio is simply not proportional to number of fraction is that more images are required for initial set-up.
Tracking method impact
While previous versions were "beam-based," the latest version of CK tracking system software is "time-based" for the number of images taken during treatment. This parameter is further adjusted with clinical variables such as patient compliance.
There was a 150% increase in the median number of images taken between skull tracking and spine tracking patients. For example, for institution B, the average ESE for brain and spine treatments were 17 cGy and 28 cGy, respectively. There were multiple explanations for this phenomenon. In general, due to complex beam placement in spinal lesions, spine tracking plans tend to have more beams and more nodes than cranial tumors. This is because spinal lesions tend to be more complex in shape mostly due to its closeness to spinal cord or other organs-at-risk and more movements in vertebra than skull. Therefore, in "beam-based" plans, spine tracking will deliver more imaging dose because more beams are required. Similarly, in "time-based" plans will deliver more imaging dose simply because the machine time is increased to accommodate the increased number of beams and nodes.
Conclusion
Image guidance has revolutionized radiotherapy. However, as more extensive imaging is incorporated into radiotherapy schemes, the dose from the imaging itself can result in substantial patient dose. In order to optimize the imaging dose which is delivered, a cohort of patients from two CK institutions which treated very different patient populations was studied. Results from both institutions clearly demonstrated the effects of fractionation scheme and tracking method upon the imaging dose in CK SRS can be substantial if not carefully managed. More frequent images would improve localizing tumor movement but ESE to normal tissue could be significantly reduced by careful management.
Indeed, while Institution B did not treat pediatric cases a small percentage of Institution A's patients were pediatric cases. Also, Institution B used defaulted setting for imaging but Institution A actively engaged monitoring for patient imaging and varied the frequency of it. In these cases, added dose to non-involved tissues is more important and should be minimized. It is imperative for the physics staff to communicate the impact of fractionation and tracking method to the prescribing physician. The authors recommend three simple yet effective improvements which should be considered by Cyberknife departments. First, there should be a baseline developed for imaging frequency according to patient's movement from previous patient's data for each site. Secondly, different imaging protocols could be implemented for pediatric, average adults, and large adults with tailored mA and exposure times based on further detailed imaging measurements. Lastly, the planner should also recognize that the machine travel time is dependent on the number of nodes when setting optimization objectives in the treatment planning system.
References
- Murphy MJ, Balter J, Balter S, BenComo JA Jr, Das IJ, Jiang SB, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med Phys. 2007; 34: 4041-4063.
- Dieterich S, Cavedon C, Chuang CF, Cohen AB, Garrett JA, Lee CL, et al. Report of AAPM TG 135: quality assurance for robotic radiosurgery. Med Phys. 2011; 38: 2914-2936.
- Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol. 2004; 14: 91-100.
- Hoogeman M, Prévost JB, Nuyttens J, Pöll J, Levendag P, Heijmen B. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: assessment by analysis of log files. Int J Radiat Oncol Biol Phys. 2009; 74: 297-303.
- Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys. 2002; 29: 334-344.
- Lim M, Villavicencio AT, Burneikiene S, Chang SD, Romanelli P, McNeely L, et al. CyberKnife radiosurgery for idiopathic trigeminal neuralgia. Neurosurg Focus. 2005; 18: E9.
- Fu D, Kuduvalli G. A fast, accurate, and automatic 2D-3D image registration for image-guided cranial radiosurgery. Med Phys. 2008; 35: 2180-2194.
- Ho AK, Fu D, Cotrutz C, Hancock SL, Chang SD, Gibbs IC, et al. A study of the accuracy of cyberknife spinal radiosurgery using skeletal structure tracking. Neurosurgery. 2007; 60: ONS147-156.
- Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo ML, Adler JR Jr, et al. An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Neurosurgery. 2004; 55: 1138-1149.
- Fürweger C, Drexler C, Kufeld M, Muacevic A, Wowra B. Advances in fiducial-free image-guidance for spinal radiosurgery with CyberKnife--a phantom study. J Appl Clin Med Phys. 2010; 12: 3446.
- Nioutsikou E, Seppenwoolde Y, Symonds-Tayler JR, Heijmen B, Evans P, Webb S, et al. Dosimetric investigation of lung tumor motion compensation with a robotic respiratory tracking system: an experimental study. Med Phys. 2008; 35: 1232-1240.