Research Article
Austin J Nucl Med Radiother. 2014;1(2): 6.
A Bivariate Model Predicts Hemispheric Lateralization of the Seizure Onset Zone on Inter- Ictal [18F]-FDG PET-CT Scans
Mehdi Djekidel1*, Li Fangyong2, Mark Michalski1, Rafay Ahmed1 and Xiaopan Yao3
1Department of Diagnostic Radiology, Yale University School of Medicine, USA
2Yale School of Public Health, Yale Center for Analytical Sciences, USA
3Section of Medical Oncology, Yale University School of Medicine, USA
*Corresponding author: Mehdi Djekidel, Department of Diagnostic Radiology, Yale University School of Medicine, USA
Received: November 01, 2014; Accepted: December 03, 2014; Published: December 08, 2014
Introduction
Epilepsy is a chronic debilitating condition for which current medical therapeutics are frequently unsuccessful and associated with significant side effects. It has a significant socioeconomic impact on the individual patient as measured by WHO (World Health Organization) population health DALYs (Disability Adjusted Life Years) as well as a prominent health care cost burden [1-4].
Antiepileptic drugs (AED's) confer a substantial long-term health care cost as well as are associated with debilitating side effects [5,6]. To add to this many epilepsy patients and young females of childbearing age, in whom a pregnancy is contemplated may be affected directly or indirectly by their epilepsy condition itself or by the adverse effects and teratogenicity related to AED's [7]. And although most epilepsy patients in a children or an adult population may respond to one or two AED's. About one third of these epilepsy patients do not respond to AED's and continue to have debilitating seizures. This group of patients where medical treatment is limited may benefit from advanced resective surgical techniques. Surgical resection of the Seizure Onset Zone (SOZ) can frequently yield good outcomes, but is underutilized worldwide. Failure to delineate the SOZ is a common limiting factor. Currently, preoperative clinical evaluation of epilepsy patients and definition of the SOZ relies heavily on functional and anatomical imaging as well as Intracranial Electrocorticography (IEC).
Non-invasive techniques, which identify different processes including anatomical lesions, metabolism, flow and intracellular biochemical changes, are utilized in identifying the SOZ and guiding IEC. Developing techniques that are simple to use may be of some benefit. Lateralization of the SOZ may at least guide the IEC phase and is felt to be crucial in many cases where functional and anatomical imaging show bilateral findings, which is not uncommon.
We propose to introduce novel simple, reproducible semi-quantitative indices extracted from a standard [18F]-FDG Brain PET scan. If used individually or in a multivariate model, they could guide pre surgical IEC evaluations of medically refractory epilepsy patients.
Methods
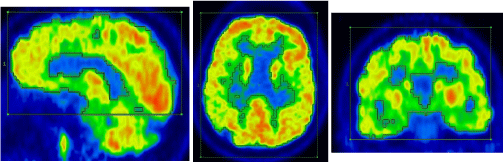
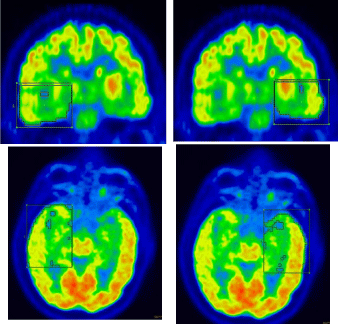
We evaluated all serial epilepsy patients with Complex Partial Seizures (CPS) whom underwent a surface electroencephalogram in the setting of an inpatient video monitoring evaluation and for whom a final lateralization of the seizure focus was determined from scalp EEG recordings and clinical semiology. A review of 72 patients evaluated during the period between 2005 and 2011 and having undergone a brain [18F]-FDG PET-CT scan was performed. 37 patients with CPS and definitive clinical and EEG lateralization were correlated and included in our analysis. Semi-quantitative parameters were analyzed including Volumes of Interest (VOI) around various structures (Global Cerebral= whole brain VOI, temporal lobes, thalami, basal ganglia, and the cerebellum) as shown in Figure 1. SUVm (maximum standardized uptake value) was used for correlation.
Figure 1 : Whole Brain, Cerebral semi-quantitative VOI measurement.
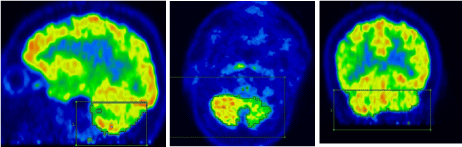
Figure 2: Cerebellar semi-quantitative VOI.
Figure 3: Temporal lobe VOI measurement.
A dichotomous value of either "Right" or "Left" was assigned to each binary variable, including global cerebral hypermetabolism SUVm, temporal SUVm asymmetry hypometabolism, thalamic hypometabolism, basal ganglia SUVm/SD asymmetry hypometabolism and cerebellum decreased visually. Surface EEG and clinical semiology lateralization served as a gold standard for the side of seizure onset. Dichotomous value "Right" was assigned to seizures lateralizing to the right and "Left" for left sided seizures. A bivariate analysis was performed for which contingency tables were constructed to present the concordance between the gold standard and each binary variable. A simple Kappa coefficient was computed to assess the agreement. Fisher's exact test was performed to examine significant associations. The predictive value of a single semi-quantitative index/ variable for each side was defined as a proportion of concordant cases among the total cases of seizure onset in that side per gold standard. Furthermore, a multivariate strategy was developed by combining information of global cerebral hypermetabolism SUVm, temporal SUVm asymmetry hypometabolism, and basal ganglia SUVm/SD asymmetry hypometabolism. The significance level was set as 0.05, two-sided. All analyses were performed using SAS 9.2 (Cary, NC).
Results
The mean age of our population was 36.5 (SD: 15.0; range: 12.9- 70.4). The mean duration of seizures since diagnosis was 14.5 years. We had 21 females and 16 males. Our population characteristics are detailed in Table 1.
Patient Characteristics
Age
36.5 ± 15.0
Years since seizure diagnosed
14.5 ± 13.1
Sex
Female
21 (59.8)
Male
16 (43.2)
Seizure Side
Left
21 (56.8)
Right
16 (43.2)
Global Cerebral Hypermetabolism SUVm
Left
20 (54.1)
Right
17 (46.0)
BG SUVm/SD Asymmetry Hypometabolism
Left
21 (56.8)
Right
14 (37.8)
No Asymmetry present
2 (5.4)
Temp SUVm Asymmetry Hypometabolism
Left
19 (51.4)
Right
16 (43.2)
No Asymmetry present
2 (5.4)
Cerebellum Down Visually
No
11 (29.7)
Yes
26 (70.3)
Thalamic Hypometabolism Asymmetry
Right
23 (62.2)
Left
12 (32.4)
No Asymmetry present
2 (5.4)
Left SUVm Basal ganglia location
Putamen
20 (54.1)
Head/Caudate/Tail
17 (45.9)
Right SUVm Basal ganglia location
Putamen
25 (67.6)
Head/Caudate/Tail
12 (32.4)
Surgery
Yes
8 (21.6)
No
29 (78.4)
Table 1: General patient characteristics. Data are presented as frequency (%) for categorical variables and mean ± SD for continuous variables.
As shown in Table 2, hemispheric cerebral hypermetabolism, determined by global cerebral hypermetabolism SUVm, was significantly associated with the contralateral side of the seizure onset (p=0.045) and showed significant moderate disagreement with seizure side with a negative kappa of -0.37 (95% CI: -0.66, -0.07). This suggests that the hemisphere contralateral to the SOZ will have the maximum uptake of FDG as determined by an SUVm measurement. If we use the right side of global cerebral hypermetabolism to predict a left sided seizure and use left to predict a right sided seizure onset, the left predictive value was 61.9%, and the right was 75.0% ,i.e, 61.9% of left sided seizures had right sided global cerebral hypermetabolism while 75% of right sided seizures had left sided global cerebral hypermetabolism.
Seizure side (Ax)
% Discordance
Left Predictive value (%)
Right Predictive value (%)
Simple Kappa a (95% CI)
Fisher's exact p value
Left
Right
% Concordance
Global Cerebral
32.4
67.6
38.1
25.0
-0.37
0.045
Hypermetabolism SUVrn, n=37
(8+4)/37
(12+13)/37
(-0.66, -0.07)
Left
8 (38.1)
12 (75.0)
Right
13 (61.9)
4 (25.0)
Basal ganglia SUVmISD
48.6
51.4
57.2
35.7
-0.07
0.737
Asymmetry Hypometabolism, n=35
(12+5)/35
(9+9)/35
(-0.4, 0.26)
Left
12 (57.2)
9 (64.3)
Right
9 (42.9)
5 (35.7)
Temporal SUVm Asymmetry
68.5
31.4
70.0
66.7
0.36
0.044
Hypometabolism, n=35
(14+10)/35
(6+5)/35
(0.05, 0.67)
Left
14 (70.0)
5 (33.3)
Right
6 (30.0)
10 (66.7)
Cerebellum Down Visually, n=37
40.5
(5+10)/3759.5
(16+6)13723.8
62.5
-0.13
(-0.41, 0.15)0.475
No
5 (23.8)
6 (37.5)
Yes
16 (76.2)
10 (62.5)
Thalamic Hypometabolism,
54.3
45.7
66.7
35.7
0.02
I.000
Asymmetry n=35
(14+5)135
(7+9)/35
(-0.30, 0.35)
Left
14 (66.7)
9 (64.3)
Right
7 (33.3)
5 (35.7)
Table 2: Agreement between seizure side and individual variables.
Temporal SUVm asymmetry hypometabolism showed significant moderate agreement with seizure side with kappa of 0.36 (95% CI: 0.05, 0.67). When temporal SUVm asymmetry hypometabolism was used to predict the side of seizure onset, we achieved a 70.0% and 66.7% (p=0.044) predictive value for left and right-sided seizures, respectively. In other words, 70.0% of left sided seizures or 66.7% of right-sided seizures was in agreement (on the same side) with temporal SUVm asymmetry hypometabolism.
Cerebellar hypometabolism was noted in 70.3% of our cases as noted through a visual assessment. Thalamic asymmetric hypometabolism was noted in 62.2% of cases but was not predictive of the seizure onset hemisphere either ipsi or contralaterally (p=1.00). Basal ganglia changes in metabolism, either increased or decreased were also not predictive of an ipsi or contralateral hemispheric seizure onset (p=0.74).
We then applied a multivariate strategy using composite variables including global cerebral hypermetabolism SUVm, temporal SUVm asymmetry hypometabolism, and basal ganglia SUVm/SD asymmetry hypometabolism (as shown in Table 3). An arbitrary post-hoc decision rule was implemented in order to explore the possible maximum predictive value that can be achieved. If the temporal SUVm asymmetry hypometabolism was on the right side, we used right side of global cerebral hypermetabolism SUVm to predict the left side of the seizure onset and the right side to predict the left side of the seizure onset. In contrast, if the temporal SUVm asymmetry hypometabolism was left sided then we predict a left side of seizure onset with the exception when all three variables were left side. For this exception, right side of seizure will be determined arbitrarily (as the red row shown in Table 3). Using this method, 80.0% and 69.2% predicted value for left and right side of seizure onset was achieved. Kappa coefficient improved to 0.49 (95% CI 0.19, 0.80) and p value was 0.01.
Temporal SUVm Asymmetry Hypometabolism
Global Cerebral Hypermetabolism SUVm
Basal ganglia SUVm/SD Asymmetry Hypometabolism
Composite index
Seizure side
Frequency
Concordance
Left
Right
Left
Left
Left
8
Left
Right
Right
Left
Left
2
Left
Left
Right
Left
Left
3
Right
Right
Right
Left
Left
2
Right
Right
Left
Left
Left
1
Right
Left
Left
Right
Right
4
Right
Left
Right
Right
Right
3
Left
Left
Left
Right
Right
2
Discordance
Right
2
Right
1
Right
1
Left
2
Left
1
Left
1
Left
Right
Left
Left
Right
2
Left
Left
Right
Left
Right
1
Right
Right
Right
Left
Right
1
Right
Left
Left
Right
Left
2
Right
Left
Right
Right
Left
1
Left
Left
Left
Right
Left
1
Seizure
side (Ax)
%
Concordance
%
Discordance
Left side predictive value (%)
Right side predictive value (%)
Simple Kappa a (95% CI)
Fisher's exact p value
Left
Right
Arbitrary rule
75.8
(16+9)/33
24.2
(4+4)/33
80.0
(16/20)
69.2
(9/13)
0.49
(0.19, 0.80)
0.010
Left
16 (80.0)
4(30.8)
Right
4 (20.0)
9 (69.2.)
Table 3: Improvement in predict value combining global cerebral hypermetabolism SUVm, temporal SUVm asymmetry hypometabolism, and basal ganglia SUVm/SD asymmetry hypometabolism.
Discussion
Functional imaging with 18F-FDG PET, 11C-Flumazenil, 11C AMT and 18F-MPPF has been able to identify the SOZ necessary to be resected [8-33]. Outcomes studies have revealed that the longer patients have epilepsy prior to surgery, the worst their outcomes. This is supported by the fact that the longer the epilepsy duration is, the more extensive the area of hypometabolism seen on [18F]-FDG PET [30,34-37]. Imaging glucose metabolism, central benzodiazepine receptors and serotonin availability has been shown to impact surgery planning and outcomes [8,9,13,16,18-25,27-29,31,34,35,38,39-62]. One of the major impediments to a wider utilization of resective surgical techniques is the challenging pre-surgical evaluation of epilepsy patients and the determination of the SOZ even in centers with extensive experience and advanced expertise. Pre- surgical evaluation frequently includes IEC in many instances to delineate the SOZ. This step is closely dependent on clinical and neuroimaging data guiding placement of the electrodes to the correct SOZ or its vicinity. Bilateral IEC is challenging and fraught with many complications, so although the ultimate goal of pre-operative investigations is to define a small area for IEC coverage, lateralization of seizures may sometimes be the only definitive deduction pre IEC placement in complicated patients. Most effective functional imaging analysis techniques use some aspect of advanced statistical parametric mapping. Either comparing patients to normal healthy control databases or different images from the same patient acquired at different time points or under different conditions. One of the challenges that can be encountered is that first these techniques are not readily available in numerous centers across the world. Secondly, they require multiple sophisticated steps (image registration, warping, subtraction and statistical analysis). A small error in any one of these steps could give erroneous results especially in non-expert hands. Finally, these advanced techniques do not always provide a clear answer and may show findings that would confound the SOZ with propagation and connectivity pathways. Developing techniques that are simpler to use may be of some benefit.
Our findings in this study are thought provoking. In pre-surgical epilepsy evaluation and IEC planning every clue and every element can be helpful. Usually epilepsy multidisciplinary surgical teams review clinical semiology, surface electrode EEG and imaging data and conclude on the seizure onset lobe area, or even hemisphere. Risks of IEC and surgery are also examined. This is further explored by IEC where a definitive SOZ is determined for surgical resection. Concordance of multiple elements' pre- IEC pointing towards a specific area is generally associated with better final localization and long- term outcomes. Our novel tool when used with currently generated techniques would be valuable. It does not require additional scans other than what is standard of care and could help especially in cases where multimodality and multidisciplinary evaluations are not conclusive or barely conclusive (and doubt remains). This would also be helpful in cases where the seizure onset area is known to be for example occipital but the side cannot be determined with certainty. Our technique may provide some assistance in lateralization. This technique using contralateral cerebral hypermetabolism and ipsilateral temporal hypometabolism to lateralize the epileptogenic zone (EZ) seems to be of same value in temporal and extra-temporal lobe epilepsy.
Limitations
Using surface EEG as a gold standard to lateralize the SOZ with inpatient video monitoring can prove difficult in nonexpert hands and may occasionally be misleading, although in expert hands EEG performs very well when it detects epileptiform activity to lateralize the seizure. Specific localization within a hemisphere is more challenging. However we did not include any patient where lateralization was not achieved. Our small sample size is also an additional limiting factor and this even though we are a large surgical epilepsy referral center and only a multicenter study would be able to further validate our findings.
The fact that only 22% of our patients underwent surgery does not allow us to examine the ultimate question of outcomes and since this is a retrospective study we cannot assess accurately the impact on IEC mapping.
Conclusion
A bivariate model using contralateral cerebral hyper metabolism and ipsilateral temporal hypo metabolism can lateralize the epileptogenic zone. This would bring another layer of information to guide pre-surgical intracranial electrocorticography studies in severe medically refractory epilepsy patients, especially in difficult cases where multimodality work-up data shows conflicting results. This needs to be further validated in a larger cohort of patients in a multicenter fashion.
References
- Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. 2002; 43 Suppl 6: 21-25.
- Burneo JG, Jette N, Theodore W, Begley C, Parko K, Thurman DJ, et al. Task Force on Disparities in Epilepsy Care; North American Commission of the International League Against Epilepsy. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia. 2009; 50: 2285-2295.
- Angalakuditi M, Angalakuditi N. A comprehensive review of the literature on epilepsy in selected countries in emerging markets. Neuropsychiatric disease and treatment. 2011; 7: 585-597.
- Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, Shafer PO, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006; 47: 1700-1722.
- Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006; 47: 1094- 1120.
- Tolman JA, Faulkner MA. Treatment options for refractory and difficult to treat seizures: focus on vigabatrin. Ther Clin Risk Manag. 2011; 7: 367-375.
- Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006; 77: 193-198.
- Didelot A, Mauguière F, Redouté J, Bouvard S, Lothe A, Reilhac A, et al. Voxel-based analysis of asymmetry index maps increases the specificity of 18F-MPPF PET abnormalities for localizing the epileptogenic zone in temporal lobe epilepsies. J Nucl Med. 2010; 51: 1732-1739.
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain. 2004; 127: 900-913.
- Assem-Hilger E, Lanzenberger R, Savli M, Wadsak W, Mitterhauser M, Mien LK, et al. Central serotonin 1A receptor binding in temporal lobe epilepsy: a [carbonyl-(11)C]WAY-100635 PET study. Epilepsy Behav. 2010; 19: 467-473.
- Meschaks A, Lindstrom P, Halldin C, Farde L, Savic I. Regional reductions in serotonin 1A receptor binding in juvenile myoclonic epilepsy. Arch Neurol. 2005; 62: 946-950.
- Filakovszky J, Gerber K, Bagdy G. A serotonin-1A receptor agonist and an N- methyl-D-aspartate receptor antagonist oppose each others effects in a genetic rat epilepsy model. Neuroscience letters. 1999; 261: 89-92.
- Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, Faillenot I, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG. Neuroimage. 2004; 22: 886-896.
- Savic I, Lindström P, Gulyás B, Halldin C, Andrée B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004; 62: 1343-1351.
- Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003; 60: 749-756.
- Didelot A, Ryvlin P, Lothe A, Merlet I, Hammers A, Mauguière F. PET imaging of brain 5-HT1A receptors in the preoperative evaluation of temporal lobe epilepsy. Brain. 2008; 131: 2751-2764.
- Lin TW, de Aburto MA, Dahlbom M, Huang LL, Marvi MM, Tang M, et al. Predicting seizure-free status for temporal lobe epilepsy patients undergoing surgery: prognostic value of quantifying maximal metabolic asymmetry extending over a specified proportion of the temporal lobe. J Nucl Med. 2007; 48: 776-782.
- Muzik O, Chugani DC, Shen C, da Silva EA, Shah J, Shah A, et al. Objective method for localization of cortical asymmetries using positron emission tomography to aid surgical resection of epileptic foci. Computer aided surgery : official journal of the International Society for Computer Aided Surgery. 1998; 3: 74-82.
- Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure. 2007; 16: 509-520.
- Kumar A, Juhasz C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010; 51: 1901-1907.
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat Rev Neurol. 2010; 6: 537-550.
- Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008; 71: 1594-1601.
- Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR Am J Neuroradiol. 2009; 30: 1811-1816.
- O'Brien TJ, Miles K, Ware R, Cook MJ, Binns DS, Hicks RJ. The cost-effective use of 18F-FDG PET in the presurgical evaluation of medically refractory focal epilepsy. J Nucl Med. 2008; 49: 931-937.
- Vinton AB, Carne R, Hicks RJ, Desmond PM, Kilpatrick C, Kaye AH, et al. The extent of resection of FDG-PET hypometabolism relates to outcome of temporal lobectomy. Brain. 2007; 130: 548-560.
- Wakamoto H, Chugani DC, Juhasz C, Muzik O, Kupsky WJ, Chugani HT. Alpha- methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr Neurol. 2008; 39: 181-188.
- Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010; 75: 2168-2175.
- Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Semin Nucl Med. 2008; 38: 227-239.
- Mazzuca M, Jambaque I, Hertz-Pannier L, Bouilleret V, Archambaud F, Caviness V, et al. 18F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. J Nucl Med. 2011; 52: 40-47.
- Akimura T, Yeh HS, Mantil JC, Privitera MD, Gartner M, Tomsick TA. Cerebral metabolism of the remote area after epilepsy surgery. Neurol Med Chir (Tokyo). 1999; 39: 16-25.
- Joo EY, Hong SB, Han HJ, Tae WS, Kim JH, Han SJ, et al. Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy. Brain. 2005; 128: 1802-1810.
- Spanaki MV, Kopylev L, DeCarli C, Gaillard WD, Liow K, Fazilat S, et al. Postoperative changes in cerebral metabolism in temporal lobe epilepsy. Arch Neurol. 2000; 57: 1447-1452.
- Muzik O, da Silva EA, Juhasz C, Chugani DC, Shah J, Nagy F, et al. Intracranial EEG versus flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000; 54: 171-179.
- Kurian M, Spinelli L, Delavelle J, Willi JP, Velazquez M, Chaves V, et al. Multimodality imaging for focus localization in pediatric pharmacoresistant epilepsy. Epileptic Disord. 2007; 9: 20-31.
- la Fougère C, Rominger A, Förster S, Geisler J, Bartenstein P. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009; 15: 50-55.
- O'Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998; 50: 445-454.
- Scheinost D, Teisseyre TZ, Distasio M, DeSalvo MN, Papademetris X, Blumenfeld H. New open-source ictal SPECT analysis method implemented in BioImage Suite. Epilepsia. 2010; 51: 703-707.
- Sood S, Chugani HT. Functional neuroimaging in the preoperative evaluation of children with drug-resistant epilepsy. Childs Nerv Syst. 2006; 22: 810-820.
- Zubal IG, Spencer SS, Imam K, Seibyl J, Smith EO, Wisniewski G, et al. Difference images calculated from ictal and interictal technetium-99m-HMPAO SPECT scans of epilepsy. J Nucl Med. 1995; 36: 684- 689.
- von Oertzen TJ, Mormann F, Urbach H, Reichmann K, Koenig R, Clusmann H, et al. Prospective use of subtraction ictal SPECT coregistered to MRI (SISCOM) in presurgical evaluation of epilepsy. Epilepsia. 2011; 52: 2239-2248.
- Matsuda H, Matsuda K, Nakamura F, Kameyama S, Masuda H, Otsuki T, et al. Contribution of subtraction ictal SPECT coregistered to MRI to epilepsy surgery: a multicenter study. Ann Nucl Med. 2009; 23: 283-291.
- Neiman ES, Noe KH, Drazkowski JF, Sirven JI, Roarke MC. Utility of subtraction ictal SPECT when video-EEG fails to distinguish atypical psychogenic and epileptic seizures Epilepsy Behav. 2009; 15: 208-212.
- Kakisaka Y, Haginoya K, Ishitobi M, Togashi N, Kitamura T, Wakusawa K, et al. Utility of subtraction ictal SPECT images in detecting focal leading activity and understanding the pathophysiology of spasms in patients with West syndrome. Epilepsy research. 2009; 83: 177- 183.
- Akman CI, Ichise M, Olsavsky A, Tikofsky RS, Van Heertum RL, Gilliam F. Epilepsy duration impacts on brain glucose metabolism in temporal lobe epilepsy: results of voxel-based mapping. Epilepsy Behav. 2010; 17: 373-380.
- Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009; 162: 972- 979.
- Takaya S, Mikuni N, Mitsueda T, Satow T, Taki J, Kinoshita M, et al. Improved cerebral function in mesial temporal lobe epilepsy after subtemporal amygdalohippocampectomy. Brain. 2009; 132: 185-194.
- Blum DE, Ehsan T, Dungan D, Karis JP, Fisher RS. Bilateral temporal hypometabolism in epilepsy. Epilepsia. 1998; 39: 651-659.
- Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, Juhász C. Clinical and histopathologic correlates of 11C-alpha-methyl-L-tryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia. 2011; 52: 1692-1698.
- Debets RM, Sadzot B, van Isselt JW, Brekelmans GJ, Meiners LC, van Huffelen AO, et al. Is 11C-flumazenil PET superior to 18FDG PET and 123I-iomazenil SPECT in presurgical evaluation of temporal lobe epilepsy? J Neurol Neurosurg Psychiatry. 1997; 62: 141-150.
- Guedj E, Aubert S, McGonigal A, Mundler O, Bartolomei F. Déjà-vu in temporal lobe epilepsy: metabolic pattern of cortical involvement in patients with normal brain MRI. Neuropsychologia. 2010; 48: 2174-2181.
- Hammers A, Koepp MJ, Labbé C, Brooks DJ, Thom M, Cunningham VJ, et al. Neocortical abnormalities of [11C]-flumazenil PET in mesial temporal lobe epilepsy. Neurology. 2001; 56: 897-906.
- Hammers A, Panagoda P, Heckemann RA, Kelsch W, Turkheimer FE, Brooks DJ, et al. [11C]Flumazenil PET in temporal lobe epilepsy: do we need an arterial input function or kinetic modeling? J Cereb Blood Flow Metab. 2008; 28: 207-216.
- Henry TR, Van Heertum RL. Positron emission tomography and single photon emission computed tomography in epilepsy care. Semin Nucl Med. 2003; 33: 88-104.
- Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008; 49: 758-764.
- Juhász C, Nagy F, Muzik O, Watson C, Shah J, Chugani HT. [11C]Flumazenil PET in patients with epilepsy with dual pathology. Epilepsia. 1999; 40: 566-574.
- Koepp MJ, Hammers A, Labbé C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000; 54: 332-339.
- Koepp MJ, Labbé C, Richardson MP, Brooks DJ, Van Paesschen W, Cunningham VJ, et al. Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain. 1997; 120 : 1865-1876.
- Koepp MJ, Richardson MP, Labbé C, Brooks DJ, Cunningham VJ, Ashburner J, et al. 11C-flumazenil PET, volumetric MRI, and quantitative pathology in mesial temporal lobe epilepsy. Neurology. 1997; 49: 764-773.
- Leeman BA, Leveroni CL, Johnson KA. Does hippocampal FDG-PET asymmetry predict verbal memory dysfunction after left temporal lobectomy? Epilepsy Behav. 2009; 16: 274-280.
- Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, et al. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008; 131: 2765-2782.
- Richardson MP, Koepp MJ, Brooks DJ, Duncan JS. 11C-flumazenil PET in neocortical epilepsy. Neurology. 1998; 51: 485-492.
- Ryvlin P, Bouvard S, Le Bars D, De Lamérie G, Grégoire MC, Kahane P, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998; 121 : 2067-2081.