
Research Article
Austin J Nucl Med Radiother. 2015;2(1): 1010.
Radiosynthesis, Biodistribution and Scintigraphic Imaging of 99mtc-Celecoxib in Experimental Rat Model of Colon Carcinogenesis
Vijayta D Chadha*, Pearl laird, Gowsia Jan and Anna Ara Khan
Centre for Nuclear Medicine, Panjab University Chandigarh, India
*Corresponding author: Vijayta Dani Chadha, Center for Nuclear Medicine (UIEAST), Panjab University, Chandigarh-160014, India
Received: November 21, 2014; Accepted: February 13, 2015; Published: February 16, 2015
Abstract
The present study radiolabeled celecoxib with 99mTc and evaluated its potential in diagnosis of experimental model of colon carcinogenesis. The radiolabeling of celecoxib with 99mTc (99mTc-celecoxib) showed 84% labeling efficiency and was found to be stable up to 4 hrs at room temperature in rat serum. The blood clearance of the 99mTc-celecoxib followed a bi-phasic release pattern whereby fast release phase was observed at 2 minutes and a slow release phase was observed after 15 minutes of drug administration. The protein binding assessed in serum was found to be 69.3%. For biodistribution studies, colon carcinogenesis was initiated through weekly subcutaneous injections of DMH (30mg/Kg body weight) for 16 weeks and the animals were dissected at 24 weeks. The biodistribution studies on control and treated animals revealed a significant percentage uptake post injection in the small intestine and the large intestine which was found to be increased significantly as a function of time. The most significant finding of the study was an increase in the uptake of the radio complex in the tumor bearing colon of rats when compared to the uptake in the colon of normal control rats. Scintigraphic images in the anterior projection from 4 hrs post injection of 99mTc-celecoxib on SPECT showed significant uptake of the radiolabeled complex in the tumor site with proven histopathological changes. The study concludes that 99mTc-celecoxib possesses selectivity towards cancerous colon tissue and can be explored further for its diagnostic potential in colon cancer detection and evaluation of treatment response.
Keywords: 99mTc-Celecoxib; Diagnostic agent; Colon carcinogenesis
Abbreviations
99mTc: Technetium-99m; DMH: 1,2-Dimethylhydrazine; SPECT: Single Photon Emission Computed Tomography; COX: cycloxygenase; FAP: Familial Adenomatous Polyposis; ITLC-SG: Instant Thin Layer Chromatography- Silica Gel; H/E: Haemotoxilin/ Eosin
Introduction
Celecoxib is a sulfa non-steroidal anti-inflammatory drug and selective COX-2 inhibitor used in the treatment of osteoarthritis, rheumatoid arthritis, acute pain, painful menstruation and menstrual symptoms, and to reduce numbers of colon and rectum polyps in patients with familial adenomatous polyposis [1-5]. Overexpression of COX-2 is an early event in tumorigenesis and has been observed in a variety of malignant tumors including colon tumors. Therefore, celecoxib, aspecific inhibitors of COX-2, received US Food and Drug Administration approval as adjunct treatment for the reduction of colorectal polyps in FAP patients. Besides FAP, celecoxib is being studied for prevention of hereditary non-polyposis colorectal cancer, sporadic colorectal adenomas, bladder cancer, actinic keratosis, and Barrett’s esophagus [6]. Further, celecoxib has shown promising role in the prevention of cancer, and has been used as an adjunct to surgery to reduce the number of adenomatous colorectal polyps in patients with the hereditary colon cancer susceptibility syndrome [7].
Since large numbers of reports indicate selective COX-2 inhibitor celecoxib to be useful for the management and prevention of cancer, its radiolabeled form could be used to diagnose the malignancies and quantitative treatment response following radiotherapy and chemotherapy in colon cancer patients. Among the various radionuclides, 99mTc has an important diagnostic role in nuclear medicine because of its readily detectable 140 keV gamma rays, and relatively short half-life of 6.01 hrs. In light of these facts, the present study was designed with an aim to radiolabel celecoxib with 99mTc and to study its biodistribution and pharmacokinetic behavior in rat model of colon carcinogenesis.
Materials and Methods
Chemicals
All the chemicals used in this study were of analytical grade. DMH and Celecoxib were procured from Sigma Aldrich Company (Delhi, India). Stannous Chloride dihydrate was purchased from QUALIGENS and ITLC-SG strips was procured from MERCK.
Animals
Male Sparque Dawley rats (n=10) in the weight range of 120g-150g were procured from the Central Animal House, Panjab University, Chandigarh. The animals were housed in polypropylene cages under hygienic conditions in the departmental animal house and were maintained on a standard laboratory pelleted feed (Ashirwaad Industries, Tirpari, and Punjab) and water ad libitum throughout the period of experimentation. All the procedures on rats were done in accordance with ethical guidelines for care and use of laboratory animals which were approved by Institutional Animal Ethics Committee (IAEC), Panjab University, Chandigarh, India.
Radiolabeling
99mTc-celecoxib was prepared by adding 200 μCi of 99mTc to a vial containing 50μl (50 μg) of celecoxib. To the mixture 50μg of stannous chloride was added and the pH was adjusted to 7- 7.5 with 0.1M NaHCO3. The contents were incubated for 1 hr at room temperature.
Radiochemical purity analysis
Percentage labeling of celecoxib with 99mTc was carried out by ascending chromatographic technique. Briefly, ITLC strips were cut into appropriate length and width and were marked at the point of origin and end line (solvent front) from the base. A single spot of preparation was applied on the strip at the point of origin. Two such strips were prepared and then placed in tubes containing acetone and a mixture of Pyridine: Acetic acid: water (3:5:1.5 v/v) as mobile phases to measure the amount of free 99mTc fraction and hydrolyzed 99mTc fraction respectively. The strips were left undisturbed to allow movement of the solvent. The strips were then removed from the developing vials and counted for activity at different section of the strip in well-type gamma-sensitive probe (ECIL, Hyderabad, India).
Serum stability and plasma protein binding of the radiocomplex
Blood samples were drawn from rats under light ether anesthesia by puncturing the retro-orbital plexus using sterilized glass capillaries. Serum was then collected for the serum stability analysis of the complex. Briefly, 100 μl of the radiocomplex (1 mCi) was incubated with 900 μl of serum at 37°C for different time intervals up to 6 hrs. The samples were applied on ITLC-SG strips and developed in 100% acetone to check for any dissociation or degradation of labeled complex.
The in vitro plasma protein binding of the complex was estimated in rat plasma by incubating 100 μl of the radiocomplex with 900 μl of plasma at 37°C up to 1hr. Then 1ml of 10% TCA was added to the complex and centrifuged at 2000 rpm for 5 min. Supernatant was then collected in a different tube, and the pellet was resuspended in 1ml of 5% TCA and centrifuged again. The supernatant was collected and radioactivity was measured in both the precipitate and supernatant fraction in a well type gamma counter. Protein binding of the complex was expressed as a fraction of radioactivity bound to protein as a % of total activity.
Blood kinetics
200μCi activity of the radiolabeled complex was injected intravenously through the penile vein of the rat. Blood was drawn at different time intervals from the ocular vein, and counted for radioactivity.
Biodistribution studies
Animals were segregated into two treatment groups. Animals in Group I served as normal controls and was administered with 1mM EDTA-saline subcutaneously per week, which was used as the vehicle for treatment in DMH treated animals. For colon tumor induction, animals in Group II were given a weekly subcutaneous injection of DMH at a dose level of 30mg/Kg body weight dissolved in 1mM EDTA-normal saline (pH-6.5), for a total duration of 16 weeks [8]. The animals were dissected after 24 weeks.
After the treatment protocol, 1ml of the radio complex was injected into the rat intravenously. All the animals are sacrificed using overdose of ether anesthesia and desired organs were removed. Each organ was weighed and counted using NaI (Tl) scintillation counter (ECIL, Hyderabad, India). The geometry was kept constant. The percentage uptake per unit mass of the organ was calculated with respect to a standard having the same activity as that injected. Colon tissue was also preserved in 10% formalin for histological studies.
Histoarchitecture of control and DMH treated colon tissue was determined by H/E staining. Fixed tissue sections of the colon were dehydrated using different grades of alcohol, parafinized and then stained with H/E stain. Stained transverse sections were examined under a light microscope for preneoplastic/neoplastic changes in DMH treated group.
Gamma imaging of 99mTcO4 celecoxib in rats
To confirm the uptake of 99mTco4-celecoxib, imaging was carried out in DMH treated animals using SPECT gamma camera. SPECT image was taken at different time intervals and each image taken was of 50K counts with image matrix of 256×256.
Results
The present study evaluated the radiolabeling of 99mTcO4 with celecoxib and also evaluated its biodistribution in animals following DMH induced colon carcinogenesis. The radiocomplex exhibited 84% labeling efficiency when subjected to ITLC at different time intervals. The radiocomplex was stable in in vitro physiological conditions as observed for 4 hrs at room temperature in rat serum (Table 1). The blood clearance of the 99mTc-celecoxib followed a biphasic release pattern whereby fast release phase was observed at 2 minutes and a slow release phase was observed after 15 minutes of drug administration (Figure 1). The protein binding assessed in serum was found to be 69.3%.
TIME INTERVAL
% binding in serum
15 min
86.8
30 min
83.4
1 hr
83.1
2 hrs
84.9
4 hrs
86.1
Table 1: Serum stability of 99mTc-Celecoxib.
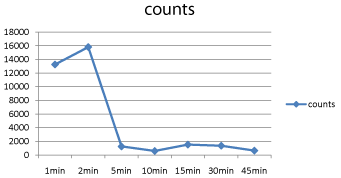
Figure 1: Blood kinetics of 99mTc-celecoxib following intravenous injection in
rats.
The histological changes in the DMH treated group confirmed neoplastic changes in the colon tissue with well differentiated signs of adenocarcinomas (Figure 2). The results (% uptake) of the in vivo biodistribution studies carried out in normal and DMH treated animals at different time intervals after injection of 99mTc-celecoxib are shown in Table 2 and 3. The study revealed highest uptake of 99mTc-celecoxib by the liver followed by kidney and spleen. Further, the small intestine and the large intestine showed a significant percentage uptake post injection which was found to be increased significantly as a function of time. The most significant finding of the study was an increased uptake of the radiocomplex in the intestine of the DMH treated rats when compared normal control rats. Further, a significant uptake was witnessed at the site of tumors when compared to the uptake at the adjacent site of tumor area.
Organ
30 min
2 hour
4 hour
Liver
33.40 ± 3.9
38.99 ± 5.8
27.44 ± 6.3
Heart
1.8 ± 0.29
2.3 ± 0.33
2.4 ± 0.32
Kidney
24.41 ± 6.2
26.77 ± 4.9
19.06 ± 3.8
Brain
0.17 ± 0 .03
0.16 ± 0.07
0.18 ± 0.08
Spleen
18.71 ± 5.1
18.04 ± 3.8
15.62 ± 4.4
Bone
0.68 ± 0.14
0.47±0.08
0.49±0.06
Muscle
0.154 ±0.019
0.053 ±0.027
0.090 ±0.054
lung
0.72 ±0.16
1.18 ± 0.25
1.21 ±0.19
Small intestine
3.32 ± 0.24
3.94 ± 0.41
3.591 ± 0.40
Large intestine
2.16 ± 0.31
2.29 ± 0.19
2.24 ± 0.28
Mean ± S.D. (Mean of three observations)
Table 2: Biodistribution of 99m Tc-celecoxib in normal control rats.
Organ
30 min
2 hour
4 hour
Liver
30.29 ± 4.4
33.67 ± 6.4
27.41 ± 4.8
Heart
1.5 ± 0.12
1.7 ± 0.19
1.34 ± 0.11
Kidney
22.11 ± 3.9
27.46 ± 5.5
20.14 ± 3.6
Brain
0.19 ± 0 .06
0.16 ± 0.03
0.19 ± 0.05
Spleen
17.66 ± 4.2
19.04 ± 5.7
14.22 ± 3.9
Bone
0.61 ± 0.13
0.53 ± 0.11
0.55 ± 0.09
Muscle
0.11 ±0.04
0.08 ± 0.03
0.09 ±0.04
lung
1.12 ±0.22
1.31 ± 0.26
1.05 ±0.18
Small intestine
5.33 ± 0.31
4.94 ± 0.40
5.19 ± 0.46
Large intestine
3.17 ± 0.22
3.93 ± 0.32
3.73 ± 0.36
Colon tumor site
1.17 ± 0.29
1.43 ± 0.31
1.39 ± 0.36
Adjacent site of tumor
0.57 ± 0.07
0.49 ± 0.06
0.59 ± 0.07
Mean ± S.D. (Mean of three observations)
Table 3: Biodistribution of 99m Tc-celecoxib in DMH treated rats.
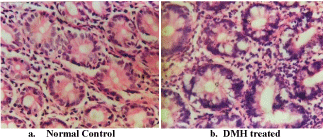
Figure 2: Microscopic images of colon tissue stained with H/E stain, a)
reveals normal histo-architecture in control group. b) Abbereant crypt foci and
dysplasia can be observed in colon tissue from DMH treated rats.
Scintigraphic images in the anterior projection from 4 hrs post injection of 99mTc-celecoxib on SPECT showed significant uptake of the radiolabeled complex in the proximal colon corresponding to the tumor site with proven histopathological changes as was done after the scan (Figure 3).
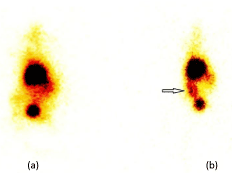
Figure 3: Planar images in the anterior projection after (a) 1 hour post
injection of 99mTc-celecoxib and (b) 4 hours post injection of 99mTc-celecoxib.
Arrow shows tumor site in the rat colon.
Discussion
In the present study, the labeling of celecoxib with 99mTc showed >84% radiolabeling, which was sufficiently stable in in vitro physiological conditions for 4 hours at room temperature. Among the conventional method of labeling, the reduced 99m Tc (v) (most probable oxidation state present in 99mTc complexes) forms stable complex with various donor groups explicitly with nitrogen sulphur and oxygen ligand. Since, celecoxib also consist of a donor group NH2 in its structure unit, it is likely that it forms coordinate bond formation with 99mTc, though further confirmative studies are still required to clearly support this assumption.
It is well known, that the percentage binding of the radiopharmaceutical with the protein has a direct impact on the biodistribution, rate of elimination and the target uptake of the radiopharmaceutical [9,10]. The protein binding of 99mTc-celecoxib assessed in serum was found to be 69.3%. It can be stipulated that because of this high protein binding of the celecoxib, there is delay in the excretion of the radiocomplex from the Reticuloendothelial System (RES) and urinary system. The blood clearance of the radiolabel in rats revealed biphasic release pattern with the peak activity seen in the blood at two time interval i.e. 2´ and 15´ post injection. The first peak at 2´ indicates fast clearance from the blood suggesting that the radio complex is quickly taken up by different organs or is being eliminated from the body at a very fast rate. The slow clearance of tracer observed in the second phase of blood kinetics is suggestive of the release of tracer from different organs into the blood pool.
The present study revealed a similar pattern of in vivo biodistribution of radiocomplex in DMH treated rats when compared to normal rats. However, there was a higher uptake of radiocomplex in the intestine of rats treated with DMH, when compared to normal control group rats, indicating better selectivity of the radiocomplex towards the intestinal tissue with preneoplastic/neoplastic histological pattern.
Histological changes in the treated group confirmed neoplastic changes in the tissue which could be responsible for the increased uptake of the radiocomplex in the DMH treated group when compared to the control group. Further, the percentage uptake was observed to peak at 2 hrs and was stable up to 4hrs, in the large intestine of DMH treated rats, whereas no significant change in the uptake is observed in normal intestinal tissue after administration of the radiopharmaceutical with time. This indicates 2-4 hrs time intervals as an ideal time to determine tumor uptake using 99mTca celecoxib. Maximum tissue activity was found in the liver, which was not significantly reduced even after 4 hrs. Since celecoxib extensively gets metabolized in the liver after administration [11], therefore, the metabolites so formed could not be excreted quickly via hepatobiliary route, resulting in increased percentage uptake of the radiocomplex in liver. Kidneys showed an increase in activity with time which peaked at 2 hrs, clearly indicating the excretion of the activity through the renal route. The major routes of excretion for celecoxib are feces and urine [12] which is however observed to be slightly delayed. This can be well corroborated from the present finding of high protein binding of the 99mTc-celecoxib, resulting in delayed excretion of the radio-complex from the kidney.
Planar SPECT images recorded from the anterior projection after 4 hrs post injection of 99mTc-celecoxib has shown increased uptake in the proximal colon corresponding to increased % age specific uptake at the site of tumor. The site of increased uptake showed neoplastic changes in the histoarchitecture as confirmed from the histopathological studies and thus suggest that the 99mTccelecoxib possesses better selectivity towards the neoplastic colon tissue of DMH treated rats when compared to normal control rats. Celecoxib is a known COX-2 inhibitor that correlates this drug with the suppression of tumor development, thus the increased uptake of 99mTc-celecoxib in the biodistribution studies and SPECT images is due to the over expressed COX-2 in neoplastic colon tissue.
The preliminary studies on the newly developed radiopharmaceutical 99mTc-celecoxib have proven its high labeling efficacy, in vitro and in vivo stability. Further, the biodistribution pattern and Scintigraphic imaging clearly suggests that 99mTccelecoxib possesses better selectivity towards the cancerous colon tissue when compared to normal colon tissue in rats and thus has a promising future to be explored further for its diagnostic potential in colon cancer detection and evaluation of treatment response.
Acknowledgement
The authors acknowledge with thanks Dr Ashok Kumar Goyal, Senior Consultant and Head, Nuclear Medicine Department, Mohan Dai Oswal Hospital, Ludhiana, for obtaining SPECT images for the present study.
References
- Clemett D, Goa KL. Celecoxib. A review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs. 2000; 59: 957–980.
- Fort J. Celecoxib, a COX-2-specific inhibitor: the clinical data. Am J Orthop (Belle Mead NJ) 1999; 28: 13–18.
- Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006; 355: 885–895.
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006; 355: 873–884.
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000; 342: 1946–1952.
- Vernon E. Steele. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer Mutation Research. 2003; 523–524: 137–144.
- Gong Li, Thorn Caroline F, Bertagnolli Monica M, Grosser Tilo, Altman Russ B, Klein Teri E. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics and genomics. 2012; 22: 310–318.
- Vijayta Dani, Ajay Goel, Vaiphei K, Dhawan DK. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicology Letters. 2007; 171: 10-18.
- Trainor GL. The importance of plasma protein binding in drug discovery. 2007; 2: 51-64.
- Jusko WJ, Gretch M. Plasma and tissue protein binding of drugs in pharmacokinetics. Drug Metabolism Review. 1976; 5: 43-140.
- Paulson SK, Hribar JD, Liu NW, Hajdu E, Bible RH, Piergies A, et al. Metabolism and excretion of [(14)C]celecoxib in healthy male volunteers. Drug Metabolism & Disposition. 2000; 28: 308–314.
- Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular risk of celecoxib in 6 randomized placebocontrolled trials: the cross trial safety analysis. Circulation. 2008; 117: 2104–2113.