
Review Article
Austin J Nucl Med Radiother. 2016; 3(1): 1014.
Hypoxia Image Guided Radiation Therapy: Current Status and Major Challenges
Hong Yuan1,2*, Zibo Li1,2 and Sha Chang³
¹Department of Radiology, University of North Carolina at Chapel Hill, USA
²Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, USA
³Department of Radiation Oncology, University of North Carolina at Chapel Hill, USA
*Corresponding author: Hong Yuan, Department of Radiology, Campus box #7513, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Received: January 20, 2016; Accepted: February 01, 2016; Published: February 03, 2016
Abstract
Tumor hypoxia is a major factor contributing to treatment resistance and local recurrence in radiotherapy. Over the years hypoxia has become a target of modulation to improve tumor control in radiotherapy, including hyperbaric oxygenation, hypoxic radiosensitizers, and in recent years, Hypoxia Image Guided Radiotherapy (HIGRT).The HIGRT is one of the biologic image guided radiotherapy methods aiming to delivering higher radiation dose to hypoxic sub-volumes to overcome hypoxia-induced radio resistance. The concept of delivering higher radiation dose to hypoxic tumor tissue became possible reality only after major developments on the non-invasive imaging on hypoxia, especially the PET imaging with hypoxia probes. Several radiation therapy treatment planning studies have been carried out with modulated radiation dose based on hypoxia PET images. However there are several practical challenges to implement the procedure in real clinical condition due to imaging limitation, physiological variation, and limitations on radiation delivery. The article reviews the current status on clinical and preclinical research on HIGRT and major challenges to be addressed in the future development.
Keywords: Hypoxia imaging; Image guided radiotherapy; Dose escalation
Abbreviations
HIGRT: Hypoxia Image Guided Radiotherapy; PET: Positron Emission Tomography; MRI: Magnetic Resonance Imaging; IMRT: Image Modulated Radiotherapy; IGRT: Image Guided Radiotherapy; TCP: Tissue Control Probability; OAR: Organ At Risk; DPBN: Dose Painting By Number; GTV: Gross Tumor Volume; HTV: Hypoxia Tumor Volume; HNSCC: Head And Neck Squamous Cell Carcinoma; SUV: Standardized Uptake Value; FMISO: 18F-Fluoromisonidazole; FAZA: 18F-Fluoroazomycin Arabinofuranoside, FETNIM: 18F-Fluoroerythronitroimidazole, EF5: 18F-[2-(2-nitro-1[H]-imidazol-1-yl)-N-(2,2,3,3,3- pentafluoropropyl)-acetamide], HX4: 18F-flortanidazole, Cu-ATSM: Cu-diacetyl-bis(N4-methylthiosemicarbazone); FLT: 3-deoxy-3- [18F]-fluorothymidine; DCE MRI: Dynamic Contrast Enhanced Magnetic Resonance Imaging.
Overview
Cancer radiotherapy today largely relies on CT and MRI images to delineate tumor volume and critical structures for treatment planning in order to attempt the optimal balance between tumor control and normal tissue sparing. However, loco regional recurrence remains to be a major obstacle for the treatment of many advanced malignant tumors [1,2]. One contributing factor of local recurrence is tumor hypoxia [1,3]. Hypoxia (low oxygenation) is a characteristic feature in most malignant tumors. Direct measurement using Eppendorf oxygen probe resulted oxygen potential less than 10mmHg with great heterogeneity within tumor in many different type of cancers including lung cancer, cervical cancer, head and neck cancer, etc. [4-6]. Tumor hypoxia has been shown to be closely related with the resistance to radiotherapy and the subsequent tumor recurrence after radiotherapy [7-9]. Over the years hypoxia has become a target of modulation in research to improve tumor control in radiotherapy using methods including hyperbaric oxygenenation [10], hypoxic radiosensitizers [11,12], and in recent years, hypoxia image guided radiotherapy (HIGRT) [13-16]. HIGRT is a biologic image modulated radiotherapy based on functional imaging of tissue hypoxia rather than anatomical structure imaging alone as commonly used in radiation therapy today.
Conventional anatomical imaging based radiotherapy delivers the same radiation dose to all regions of the tumor volume regardless of their radio sensitivities, potentially leaving hypoxia-induced radio resistant cancer cells surviving the radiation. Recent development of hypoxia imaging technique has made the development of HIGRT, especially the rapid development on hypoxia PET imaging which provides spatial distribution and magnitude of tissue oxygenation non-invasively. With hypoxia imaging, HIGRT can utilize hypoxia information to spatially modulate the radiation dose distribution so that higher radiation dose is delivered to hypoxic tumor cells without compromising normal tissue sparing. The goal of HIGRT is to overcome the hypoxia-induced radio resistance, thus enhance radiation therapeutic ratio [17,18].
The first attempt of introducing hypoxia image in radiation planning started about 15 years ago [19]. Chao et al first conducted a feasibility radiation planning study with the guidance from hypoxia PET imaging with 60Cu-ATSM. The study demonstrated that radiation dose can be escalated in the hypoxia sub-volume defined by the 60Cu-ATSM hypoxia images without increased dose on normal tissue. More treatment planning studies have been published since then, however there have been no reports on actual delivery of HIGRT on clinical patients yet. This article will review the current research status both on preclinical and clinical level, and discuss major challenges the HIGRT field faces today.
Current Status
There are two major trends on the HIGRT approaches. One is to define the hypoxic volume by segmenting a volume based on threshold criteria on hypoxia images and deliver a uniform boosting dose to the hypoxia sub-volume [17,19-21]. Another approach is so called Dose Painting by Numbers ((DPBN) Figure 1). The idea of hypoxia DPBN is to use the spatial distribution of hypoxia provided by the PET image directly and apply spatially variant doses according to the degree of the hypoxia, i.e. higher dose in hypoxic foci and lower dose in well oxygenated tissue [22-24]. Both approaches are still under early stage of radiation planning studies, and theoretical simulation studies have been conducted to predict and evaluate the treatment outcomes from HIGRT.
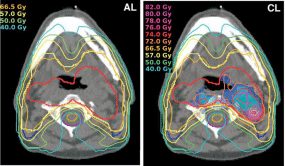
Figure 1: DPBN radiation treatment planning. Left: Conventional IMRT based
on CT image; Right: Dose painting based on FMISO hypoxia PET images.
Volume within Red line: primary target volume; volume within Pink line:
hypoxic volume. (Modified from [22]).
Clinical study
According to the website Clinicaltrials.gov, there are seven clinical trials (five active) registered with focus on hypoxia image based radiotherapy. These trails are carried out in various tumor types, including chordoma; prostate adenocarcinoma, Head and Neck Squamous Cell Carcinomas (HNSCC), and Non-Small Cell Lung Cancer (NSCLC). All the trials propose to use PET imaging with FMISO for hypoxia imaging. Although no patient outcomes from the HIGRT have been reported, several radiation treatment planning studies have been published and shown that dose escalation to hypoxia sub-volumes is technically feasible and can improve therapeutic ratio as shown in radiobiological modeling study. (Table 1) summarizes major planning studies on clinical patient data using hypoxia image for dose escalation and dose painting.
Authors
Year
Patient number
Tumor type
Hypoxia probe
Hypoxia Segmentation Criteria
Escalated Dose (Gy)
Evaluation
Chao CK, et al [19]
2001
NA
HNSCC
Cu-ATSM
TMR>2
80
Planning study, no TCP modeling
Thorwarth D., et al [22]
2007
12
HNSCC
FMISO
DPBN
Up to 20% boost
Mean 14.3% increase on TCP
Grosu AL, et al [16]
2007
18
HNSCC
FAZA
TMR>1.5
80
Planning study, no TCP modeling
Lin Z, et al [86]
2008
7
HNSCC
FMISO
TBR>1.3
84
Planning study, no TCP modeling
Lee NY, et al [20]
2008
10
HNSCC
FMISO
TBR>1.3
84
Planning study, no TCP modeling
Bowen SR, et al [23]
2009
3
HNSCC
Cu-ATSM
DPBN
Up to 90 Gy
Planning study, no TCP modeling
choi W, et al [95]
2010
8
HNSCC
FMISO
Tumor/cerebellum ratio >1.3
78
Planning study, no TCP modeling
Hendrickson K, et al [21]
2011
10
HNSCC
FMISO
NA
80-90
Mean 17% increase on TCP
Toma-Dasu I, et al[24]
2012
7
HNSCC
FMISO
4 regions based on PO2 map
Up to 121 Gy
Only Planning study, no TCP modeling
Chang JH, et al [25]
2013
8
HNSCC
FMISO
TMR>1.5
84
Mean 20% increase on TCP, without changes on NTCP
Henriques D, et al [96]
2014
20
HNSCC
FMISO
Adaptive Bayesian Segmentation
79.8
18.1% increase on TCP, 4.6% increase on parotids NTCP
Even AJ, et al [17]
2015
10
NSCLC
HX4
TBR >1.4
Up to 129 Gy
Planning study, no TCP modeling
Servagi-Vernat S, et al [97]
2015
12
HNSCC
FAZA
>bkg mean + 3SD
86
Planning study, no TCP modeling
TMR: Tumor to Muscle Ratio; TBR: Tumor to Blood Ratio; DPBN: Dose Paining by Numbers; SD: Standard Deviation; TCP: Tumor Control Probability; NA: data not available.
Table 1: Hypoxia image guided radiation treatment planning study with clinical imaging data.
Most of studies utilize the strategy of dose escalation on hypoxia sub-volume. In the study by Chao et al. hypoxia volume was determined by a threshold that was two times higher of the SUV in contra lateral normal muscle tissue [19]. The prescription of 80Gy in 35 fractions to the 60Cu-ATSM positive hypoxia target volume was chosen arbitrarily and the remaining tumor volume receives 70Gy. The organs at risk such as parotid grands only received less than 30Gy, demonstrating uncompromised normal tissue sparing. In 2013 Chang et al. reported another HIGRT study using FMISO hypoxia PET imaging on eight HNSCC patients [25]. Three radiotherapy plans were created for each patient: a standard (STD) plan, a Uniform Dose Escalation (UDE) on the standard Gross Tumor Volume (GTV), and a Hypoxia Dose-Escalation (HDE) plan on hypoxia sub-volume. Hypoxia sub-volumes were defined as tumor tissue that has tumor to muscle ratio of 1.5 or higher. The therapeutic effects were evaluated by a biological modeling on TCP and Normal Tissue Complication Probability (NTCP). Results from evaluation modeling showed that the mean TCPs were 73%, 94%, and 93% for STD, UDE, and HDE plans, respectively, and the mean parotid NTCP increased about 22% for UDE plan, but almost the same for HDE plan compared to STD plan, indicating enhanced therapeutic ratio for HDE plan [25]. More recently, Even et al conducted dose escalation study based on metabolic sub-volume from FDG PET and hypoxia sub-volume obtained from 18F-HX4 hypoxia PET imaging [17]. Radiation dose was escalated to 117 ± 15 Gyinhypoxia sub volume [17]. It was demonstrated that high dose to hypoxia volume can be achieved without increasing dose to organs at risk.
Different from uniform dose escalation, Alber et al. [14] and Bentzen [26] proposed the more sophisticated DPBN method, where radiation dose varies based on the hypoxia mapping within tumor volume. Thorwarthet al conducted a DPBN planning study on thirteen HNSCC patients [22]. In that study, three different treatment plans were created for each patient: conventional IMRT, a uniform 10% dose escalation to the Fluorodeoxyglucose (FDG) positive volume, and the DPBN plan based on 18F-FMISO PET images. In DPBN plan, the dose escalation factor map was determined based on dynamic FMISO PET imaging and used for a gradual dose prescription with the maximum dose per fraction limited to 2.4Gy [27]. An example of dose planning was shown in (Figure 2). The spatial resolution of the dose-escalation map was given by the PET voxel size of 4.0 x 4.0 x 4.25mm³. Their results showed a potential increase of TCP from 55.9% in conventional IMRT scheme to 70.2% in DPBN without increasing the NTCP.
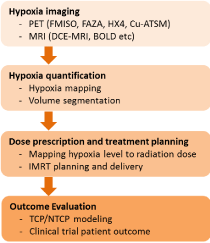
Figure 2: Elements in Hypoxia Image Guided Radiotherapy (HIGRT) study.
To this end, there has been no report on actual radiation delivery based on hypoxia imaging on clinical patients although several clinical trials have been undertaking. Treatment outcome evaluation from clinical trials is highly critical to advance the HIGRT technique. Before clinical outcome evaluation becomes possible, thorough validation on treatment plan using radiobiological modeling should be always included in planning studies.
Preclinical study
There were much fewer animal studies on HIGRT compared to clinical studies. This is mainly due to the technical limitation of targeting high radiation dose to small hypoxia region at mouse scale with high accuracy. Christian et al. compared the FDG images from animal PET scanner (2.7mm resolution) and autoradiography (100 micron resolution) in two tumor mouse models, and found that the hyper-metabolism volumes segmented from PET images had only 39% matching volume with the images from autoradiography [28]. Due to the relative low resolution of animal PET system compared to the mouse structure, achieving accurate spatial distribution of hypoxia within animal tumor model is very limited.
Nevertheless many preclinical studies have demonstrated the close correlation between tumor hypoxia and radiation treatment response [29,30]. Schutze et al. conducted a stratified dose escalation study on FaDu SCC tumor of mouse model [31]. In that study, animals were divided into two groups for 25Gy and 35Gy of single dose radiation, setting 10Gy of dose escalation. FMISO PET imaging was used to delineate the hypoxia level within tumor before radiation treatment. Although the study did not apply escalated dose within animal tumor based on the hypoxia level it has demonstrated the negative effect of hypoxia on local tumor control and escalated radiation dose could enhance the outcome in hypoxic tumor group.
Today HIGRT technology for animal research is not widely available although new commercial image-guided small animal irradiators (developed by Precision X-ray and Xstrahl Life Sciences) have the IGRT capability with targeting precision up to 0.1 mm. These systems could open up more HIGRT studies on small animals. On the other hand, well-designed HIGRT studies on large animal models with spontaneous tumor would be highly valuable to evaluate treatment outcomes with different dose schemes, and provide in depth information on HIGRT relevant to clinical trials.
Major Challenges in HIGRT
It is conceptually rational to deliver more dose of radiation to the higher hypoxia region to achieve the needed tumor control. However, HIGRT poses a number of challenges to execute the concept in reality. There are at least three key required elements for a successful implementation of HIGRT: 1) A reliable hypoxia imaging method with high accuracy, specificity and sensibility; 2) A verified model to connect the hypoxia image to radiation dose prescription; 3) A delivery device and controller that can accurately deliver accurate radiation dose to specified location. (Figure 2) shows the main elements in a HIGRT study. In addition to the above requirements, there are many biological questions remain unclear. How tumor hypoxia changes after radiation? Is single hypoxia image enough or multiple imaging sessions are necessary for adequate treatment? More research studies are needed to clarify these biological questions and meanwhile to improve the needed technical skills. The following sections are aimed to detail these needs and questions.
Hypoxia imaging and imaging probe
HIGRT is one of biologic image modulated radiotherapy methods based on functional imaging of tissue hypoxia rather than anatomical structure imaging only. This requires that the functional imaging to be sensitive and specific enough to tissue hypoxia. There have been several imaging techniques developed for tissue hypoxia imaging, including PET imaging with hypoxia probes [32], MR imaging method [33], EPR method [34], and optical imaging [35]. A more complete review on hypoxia imaging technique can be found in these reviews [36,37]. Among these techniques, PET imaging with hypoxia probes is the most promising and widely used in many studies. With the first hypoxia PET probe, FMISO, being tested on animal in 1986 [38], a number of hypoxia PET probes have been developed and evaluated, including F-18 radiolabeled FMISO [39], FAZA [40], FETNIM [41], EF5 [42], HX4 [43], Ga-68 radiolabeled nitroimidazole derivatives [44], and Cu-60 or Cu-64 radiolabeled Cu-ATSM [45]. Except Cu-ATSM, most hypoxia markers are nitroimidazole related compounds.
Among all the PET hypoxia probes, FMISO is the most extensively studied and widely used PET radiotracer for imaging tumor hypoxia. The mechanism of its hypoxia selectivity has been well studied [46,47]. When FMISO diffuses into cells, it is first reduced by nitro reductase enzymes to a radical form. Under aerobic conditions with abundant oxygen, the radical compounds will be reoxidized and diffused out of cells. However, in hypoxic condition, these radicals will be bound to intracellular macromolecules and get accumulated inside cells. Almost all nitroimidazole derivative compounds share the similar mechanisms of retention and accumulation in hypoxic tissue. Studies have shown good hypoxia specificity of FMISO with good correlation between direct PO2 measurement and FMISO retention [48,49]. The binding of FMISO to hypoxic cells mostly occurs when PO2 is between 2 to 10 mmHg [48,50]. FMISO PET imaging has gone through many clinical trials with different type of cancers, including HNSCC [51,52], NSCLC [53], soft tissue sarcoma [54], renal tumor [55], and brain tumor [56]. It has reported that there is strong correlation between FMISO uptake as hypoxia indicator and the therapeutic outcomes [53,57,58]. However, the major problem with FMISO is its slow blood clearance and its relatively low tumor-to-blood ratio.
FAZA and FETNIM, and more recently developed HX4 probe have much better hydrophilicity and thus shorter blood clearance time, leading to shorter circulation time and better tumor to normal tissue contrast ratio compared to FMISO [40,43,59-62]. However, even with HX4 probe with better hydrophilicity, Zegers et al. reported that PET imaging at 4 h is superior to 2hours post injection on nonsmall cell lung cancer patients. In general, nitroimidazole probes need average 2-4 hours waiting time after injection to reach optimal contrast and stability.
Unlike the nitroimidazole group, metal chelated compound, such as Cu-ATSM has much faster blood clearance time, and provides superior tumor uptake contrast [45,63,64]. Intracellular Cu-ATSM has been shown to be bioreduced and trapped in viable hypoxic cells quickly within 30min with high tumor to muscle ratio above two [65]. However, Cu-ATSM suffers from its nonspecific binding independent of tissue hypoxia as reported by several studies [66-68]. Additional studies that further delineate its retention mechanism will be needed before its wide clinical usage. To date, there has not been a single hypoxia probe that is considered as the gold standard, which poses problems on interpolation and quantification of hypoxia imaging using different hypoxia probes.
A separate imaging related issue is the imaging resolution capability. Hypoxia is known to have substantial spatial variation, with steep oxygen gradients demonstrated over several cells in tens of micrometer, which is beyond the resolution of any non-invasive in vivo imaging modality, as demonstrated in (Figure 3). This means that we will not be able to capture true hypoxia distribution on cellular scale with any in vivo imaging modality [25]. The question is whether we need such high resolution imaging capability, and whether current PET imaging resolution is good enough to estimate the density and distribution of hypoxia cells on tissue scale, enabling escalated dose delivered to the hypoxic region, but not single cells. There is no answer to these questions, and we will need more clinical trial studies to develop the protocol and evaluate treatment outcomes of HIGRT.
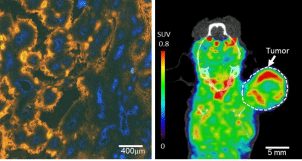
Figure 3: Microscopic image of hypoxia and hypoxia PET image on animal
tumor model. Left: Hypoxia stained by pimonidysole (orange) and vessels
stained by Hoechst 33342 (Blue), demonstrating diffusion limited hypoxia
in tumor with large heterogeneous distribution. Right: 18F-FMISO PET
imaging on tumor bearing animal, image resolution of 1.2mm, voxel size:
0.38x0.38x0.76 mm.
Hypoxia quantification
A quantitative relation between image intensity and oxygenation level is needed to interpolate hypoxia images and further prescribe radiation dose based on tissue oxygenation. Although several models have been proposed to either quantify absolute oxygen potential or segment hypoxia volumes, there has not been a well-accepted model to quantify hypoxia level and form a tissue oxygenation map based on images [24,27,69,70].
Using reference binding assay, Evans et al created a standard calibration curve between EF5 binding and absolute oxygen potential to quantify absolute tissue oxygenation based on EF5 immuno staining [71]. Such standard calibration curve has not been established for PET images due to the much more complicated nature of in vivo distribution and uptake of hypoxia imaging agents. Toma- Dasu et al. proposed the conversion of PET image intensity to tissue oxygenation using biological model fittin [72]. The same group later proposed a novel approach to obtain absolute levels of hypoxia from PET images through the use of electron paramagnetic resonance (EPR) oximetry which can provide absolute tissue oxygenation measurement [70]. The EPR method is promising because it can be used for basically any hypoxia probes for PET imaging, as long as the imaging protocol used for calibration is kept the same as used in actual hypoxia imaging. Another group used DCE MRI to create the PO2 map with the assumption that the contrast concentration is proportional to blood perfusion and thus arterial oxygen potential [69]. The major limitation of this approach is the disconnection between vascular perfusion and tumor oxygen consumption, and is not a true hypoxia representation on tissue level.
An alternative approach to calculating absolute oxygen potential is to generate indirect oxygenation-dependent parameters. Thorwarth et al used compartmental modeling on dynamic PET imaging with FMISO to generate a parametric hypoxia maps for potential dose paining [22,27]. The fundamental concept was that the vascular perfusion and uptake rate constants that govern the shape of the tissue uptake curves can be used to stratify tumor tissue to be wellperfused, hypoxic, or necrotic regions. They have found that shape characteristics on tissue uptake curves are more accurate to depict hypoxia tissue than the single SUV measurement in static imaging [27]. More validation studies are needed to evaluate the relationship between the extracted parameters and tumor radio resistance in order to use the parametric map for image guided radiotherapy.
Instead of determining hypoxia level for each voxel, another method for hypoxia quantification is to segment hypoxia volume based on certain criteria. The escalated radiation dose can be then applied to the defined hypoxic volume. The key issue here is the segmentation criteria. In this approach, Tumor-To-Blood Ratio (TBR) analysis has been mostly used to classify hypoxic tissue. Rasey et al. defined the hypoxic tumor volume with a TBR of1.4 or higher from FMISO PET image data acquired between 120 and 160min after injection [73]. The use of TBR of 1.4 as cutoff line was based on previous animal studies that ninety percent of presumed normoxic tissues had a TBR less than 1.31, and thus the ratio of 1.4 was taken as a conservative estimate for hypoxic tissue [39,73]. Rajendran JG [52] showed that TBR of 1.2 can be effective to delineate tumor hypoxia volume. Another study used the TBR threshold of 1.5 to define the hypoxia volume because the delayed imaging time at 4 hours, compared to 2-3 hours, after injection could enhance tumor to normal tissue contrast [74]. However, the TBR methodrequires blood sampling, which is invasive and could introduce technical errors and variations. An alternative method is using surrogate tissue, such as muscle, heart, or cerebellum to derive tumor-to-normal tissue ratio [74-76]. Muzi et al conducted the comparison between TBR and other tissue ratio and results showed that image derived regions can be effectively used to estimate blood activity [75]. Although it is promising to use image-derived tissue instead of blood sample, more evaluation studies are highly needed to assess the variation, accuracy, and robustness.
Radiation dose planning and delivery
In theory, HIGRT treatment dose in each sub-regions of the tumor is modulated by the hypoxia level of the tissue so that cancer cells receive the same tumor control independent of its hypoxia-related radio sensitivity. However, the relationship that maps quantitative hypoxia level to an optimal dose prescription has not been fully developed yet, due in principle to still elusive interactions between radiation and tissue responses with many biological phenotypes including proliferation, angiogenesis, hypoxia and necrosis [23]. Hypoxic cells are invariably more resistant to radiation, specifically for low linear energy transfer (LET) x-rays and gamma-rays, than welloxygenated cells. A parameter to describe such radio sensitization effect is called Oxygen Enhanced Ratio (OER). A prevalent OER for in vitro cultured mammalian cells under X-ray radiation is between 2.5- 3 fold, that is, well-oxygenated cells can have 2.5-3 times higher cell killing by radiation compared to anoxic cells [77,78]. The situation in an in vivo tumor is clearly more complicated than that in the in vitro model [79]. Based on clinical data collected from prostate cancer patients, Wang et al estimated dose escalation to overcome hypoxia for prostate tumors is 165Gy for permanent I-125 implants and 88 Gy in 2 Gy fractions for external-beam radiotherapy [80].
In most clinical studies reported, dose escalation has ranged from 15% to 50%. In the study reported by Toma-Dasu et al. the Dose Modification Factor (DMF) for each voxel was calculated based on the local oxygen tension and the maximum OER, and the prescribed radiation dose ranged from 68 to 121Gy with targeted TCP to be 95% [24]. Chang et al. boosted radiation dose from 70Gy to 84Gy on the hypoxia sub volume based on their Monte Carlo modeling study which shows that 120-150% of the dose is required to negate the detrimental effects of hypoxia on the tumor control [25].
Biological modeling of TCP and NTCP based on radiobiology parameters and their relationships played a critical role in evaluating treatment planning, before actual clinical patient outcome data are available. Several TCP models incorporating different levels of radio sensitivity to account for tumor hypoxia have been reported [22,69,77,81]. Popple et al. used Monte Carlo model to investigate the TCP under different boosting dose and hypoxia fraction [79]. They reported that modest boosting of 120%-150% increased TCP to the equivalent level in non-hypoxic tissue. However, they pointed that the improvement on the TCP was shown significant only if there is a significant portion of stable hypoxia volume [79]. Tome and Fowler have examined the boosting of sub volumes for tumors in which the radio sensitivity of the colognes varies throughout the volume [82]. They conclude that modest boost doses to an arbitrary sub volume of the tumor produce an improvement in TCP, and to obtain a significant improvement, approximately 50% or more of the volume must receive the boost dose. Almost all the models used for dose calculation are based on linear-quadratic model obtained from in vitro radio-sensitivity curves in oxygenated and hypoxic condition. Due to the various individual models, it is difficult in evaluating and comparing different strategies in current status. In addition, any mathematical modeling is a simplistic version of complicated realistic process in tumor control and normal tissue toxicity. As such, these results need to be proven in a prospective clinical trial before any firm conclusions can be drawn.
Different than other challenges in HIGRT, treatment delivery is relatively straight forward as it can be achieved using technologies already under wide clinical use. HIGRT can be delivered by the mature Intensity-Modulated Radiation Therapy (IMRT) delivery technology that has been used for more than a decade in US and many other countries [83-85]. In IMRT x-ray intensity distribution is spatially modulated/optimized to produce the intended anatomical structure based volumetric dose specification. Typical IMRT treatment delivery time depends on the specific delivery technology used and it can be from 2min to 20min.The spatial resolution requirement of a typical IMRT is in the order of 1-2mm, which should be adequate for PETbased hypoxia imaging (resolution 4-5mm). The smallest dimension of an IMRT segment field is 5mm thus the IMRT treatment delivery approach can be readily used for HGRT. For preclinical research, there are several commercial systems available for small animal image guided radiotherapy with 1mm of smallest collimator size and up to 0.1mm as image guided targeting precision. The major limitation on dose delivery lies on the hypoxia PET imaging resolution [28].
Other imaging modality such as MRI could provide much better image resolution, however the sensitivity and specificity could be compromised on the other side.
The dose treatment planning and delivery towards HIGRT is still in an early development stage. The relationship between hypoxia images and dose escalation plan is yet to be established for various tumor types. And outcome evaluation is awaiting data from perspective clinical trials.
Hypoxia temporal variation
Another major challenge in HIGRT is the change of tumor hypoxia before and during treatment. In theory, only hypoxia status at the very time of radiation delivery affects the radio sensitivity of the irradiated cell. Hypoxia temporal variation between the imaging time and radiation delivery time could potentially affect the efficacy of HIGRT.
If tumor hypoxia patterns changes significantly between the time of radiation treatment and the imaging time, the HIGRT may boost the dose to the wrong regions. Several studies have been done to evaluate the repeatability of hypoxia imaging before radiation treatment. Grosu et al. [16] has gathered evidence from animal studies that the FAZA uptake in untreated EMT6 tumors is highly correlated between two PET scans taken within 1 day. Okamoto et al. [74] conducted 2 serial FMISO scans with a two-day interval in head & neck cancer patients before treatment, and found no significant changes on SUV max, Tumor-blood-ratio, Tumor-muscle-ratio, and segmented hypoxia volume, and the correlation coefficient to be 0.959 for SUV max. This indicates the reproducibility of hypoxia PET measurements, as well as the stability of tumor tissue hypoxia over a short period.
On the other side, Lin et al investigated the changes of FMISO uptake in two sequential PET scans with three days apart, and reported that half of patients have significant changes on hypoxia distribution which could make the dose painting radiotherapy more challenging [86]. Nehmeh et al. also conducted two 18F-FMSIO PET scan with three days apart, found similar problems on hypoxia variation [87]. Their group also observed that small changes in the threshold level could have a considerable effect on the correlation analysis. However in their study, large variation on PET starting time after FMISO injection (114min-195min) and variation on blood sampling time (up to 60min difference) could induce high variation on FMISO uptake level.
More recently, Zegers et al. conducted a multicenter clinical trial with serial 18F-HX4 PET imaging on head & neck and lung cancer patients. They reported highly correlated hypoxia measurements (SUV mean, SUV max, TBR, and segmented hypoxia volume) between two scans in one week apart. The study concluded that 18FHX4 PET imaging can provide reproducible and stable results in patients with head and neck cancer and patients with lung cancer. Although the degree of hypoxia variation before radiation treatment could be highly dependent on the time interval, tumor types, and imaging protocols, more evidences have shown that variation of hypoxia before treatment might be acceptable and hypoxia imaging taken short time, at least two days before radiotherapy could be used for image guidance.
More concern is put on the variation of hypoxia during fractionated radiotherapy. Kempf et al. created a mathematical model to explore the spatial and temporal changes on tissue oxygenation after radiotherapy [88]. They observed maximum reoxygenation reached at 15hours after a single dose of 4Gy radiation, and depleted after 19 hours of radiation due to strong regrowth. Eschmann et al. conducted serial FMISO scans on HNSCC patients before and during fractioned radiotherapy. The study also showed decreased hypoxia in 12 out of 14 patients, reflecting reoxygenation after radiation treatment [89]. Controversially, Fatema et al. reported no significant changes on intratumoral FMISO uptake between control and radiated mice (10 or 20 Gy) at 6,24, and 48hours post radiation on FaDu xenograft mouse model [90]. It is worth pointing out that the comparison was done between two groups, but not on the same animal over time. Similarly, Bradshow et al conducted serial PET scans with Cu-ATSM and FLT before and during radiation fractions on canine sarcomas and carcinomas, and reported that spatial distribution of Cu-ATSM uptake were quite stable with high correlations between scans before and in the middle of treatment for both tumor types [91].
To this end, there are no conclusive results on hypoxia changes during radiation fractions. A possible approach to the dynamic nature of hypoxia would be conducting multiple hypoxias PET scans during treatment. Thus the adaptive HIGRT could ensure the best treatment outcome and that adjacent sensitive normal tissue such as mucosas do not become overdosed. Thorwarth developed the TCP model that included the changes of local oxygenation, and locally varying dose escalation factor can be used for radiotherapy planning [92]. Sovik reported significantly improved TCP by repeated replanning during the course of fractionated treatment based on repeated DCE MRI [93].
Although adaptive HIGRT with multiple hypoxia PET scans during radiotherapy are promising, many questions remain unsolved in practical implementation. Future studies are needed to determine hypoxia dynamics on different tumor types, re-oxygenation and rehypoxia dynamics over treatment period, interaction between spatial variation and temporal changes, etc. In addition, optimal imaging interval time needs to be explored to find the balance between hypoxia variation and radiation exposure from multiple PET scans.
Conclusion
Beyond the major challenges discussed above, other technical issues also exist although with less severity, such as accuracy on image registration between hypoxia image and planning CT or MRI images, and motion effects during radiation delivery. Clinical implementation of HIGRT depends on advances in all aspects of the entire process, including standardized imaging protocol, accurate quantification of functional images, improvements in delivery techniques over smaller spatial scales, treatment outcome evaluation plan, and many other factors. Though still in the early development stage, the HIGRT has been considered as one working direction in radiation oncology.
To guide radiation therapy treatment by tumor hypoxia, a relationship between treatment outcome and the considered hypoxia imaging method needs to be evaluated through clinical trials. However, to this end there has been no clinical data reporting treatment outcome evaluation on HIGRT. Results from clinical implementation of HIGRT are expected to start to merge in publications in the near future. Such data will be essential in advancing the technique of HIGRT and improving practical implementation. On the other hand, it is also important to design solid randomized clinical trials so that the treatment outcomes from HIGRT are compared to the standard radiotherapy with the equal mean tumor dose [94]. Without equal tumor dose comparison, the treatment outcome could be biased to overestimate the benefit from HIGRT, unless there is sufficient longitudinal data to address normal tissue toxicity or sparing effects.
With the progress on molecular imaging and cancer biology, the radiation treatment will be more tailored towards individual biological pattern for optical prescription and delivery of radiation. Hypoxia as one of the important prognosis markers in cancer therapy should be undoubtedly utilized for biologically optimized radiation therapy. It would bring revolutionary progress in radiation oncology if we could truly implement the concept of HIGRT. More research studies including both clinical and preclinical levels are needed to evaluate the approach of HIGRT.
References
- Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002; 7: 492-508.
- Gomez-Millan J, Lara MF, Correa Generoso R, Perez-Rozos A, Lupianez- Perez Y, Medina Carmona JA. Advances in the treatment of prostate cancer with radiotherapy. Crit Rev Oncol Hematol. 2015; 95: 144-153.
- Harada H. How can we overcome tumor hypoxia in radiation therapy? Journal of radiation research. 2011; 52: 545-556.
- Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996; 56: 4509- 4515.
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005; 77: 18-24.
- Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage nonsmall cell lung cancers. Clin Cancer Res. 2006; 12: 1507-1514.
- Karakashev SV, Reginato MJ. Progress toward overcoming hypoxia-induced resistance to solid tumor therapy. Cancer management and research. 2015; 7: 253-264.
- Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999; 53: 113-117.
- Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000; 57: 39-43.
- Bennett M, Feldmeier J, Smee R, Milross C. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst Rev. 2005.
- Hay MP, Hicks KO, Wang J. Hypoxia-directed drug strategies to target the tumor microenvironment. Adv Exp Med Biol. 2014; 772: 111-145.
- Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011; 17: 2997-3004.
- Lee NY, Le QT. New developments in radiation therapy for head and neck cancer: intensity-modulated radiation therapy and hypoxia targeting. Semin Oncol. 2008; 35: 236-250.
- Alber M, Paulsen F, Eschmann SM, Machulla HJ. On biologically conformal boost dose optimization. Phys Med Biol. 2003; 48: N31-N35.
- Hoskin PJ. Hypoxia dose painting in prostate and cervix cancer. Acta Oncol. 2015; 54: 1259-1262.
- Grosu AL, Souvatzoglou M, Roper B, Dobritz M, Wiedenmann N, Jacob V, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007; 69: 541-551.
- Even AJ, van der Stoep J, Zegers CM, Reymen B, Troost EG, Lambin P, et al. PET-based dose painting in non-small cell lung cancer: Comparing uniform dose escalation with boosting hypoxic and metabolically active sub-volumes. Radiother Oncol. 2015; 116: 281-286.
- Grau C, Hoyer M, Alber M, Overgaard J, Lindegaard JC, Muren LP. Biologyguided adaptive radiotherapy (BiGART)- more than a vision? Acta Oncol. 2013; 52: 1243-1247.
- Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001; 49:1171-1182.
- Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, et al. Fluorine- 18-labeled fluoromisonidazole positron emission and computed tomographyguided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2008; 70: 12-13.
- Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: potential for guiding intensity modulated radiation therapy in overcoming hypoxiainduced treatment resistance. Radiother Oncol. 2011; 101: 369-375.
- Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys. 2007; 68: 291-300.
- Bowen SR, Flynn RT, Bentzen SM, Jeraj R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol. 2009; 54: 1483-1501.
- Toma-Dasu I, Uhrdin J, Antonovic L, Dasu A, Nuyts S, Dirix P, et al. Dose prescription and treatment planning based on FMISO-PET hypoxia. Acta Oncol. 2012; 51: 222-230.
- Chang JH, Wada M, Anderson NJ, Lim Joon D, Lee ST, Gong SJ, et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using (18) F-FMISO PET: a biological modeling study. Acta Oncol. 2013; 52: 1723- 1729.
- Bentzen SM. The agnostic imaging for radiation oncology: dose-painting by numbers. The lancet oncology. 2005; 6: 112-117.
- Thorwarth D, Eschmann SM, Paulsen F, Alber M. A kinetic model for dynamic [18F]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol. 2005; 50: 2209-2224.
- Christian N, Lee JA, Bol A, De Bast M, Jordan B, Gregoire V. The limitation of PET imaging for biological adaptive-IMRT assessed in animal models. Radiother Oncol. 2009; 91: 101-106.
- Evans SM, Jenkins WT, Joiner B, Lord EM, Koch CJ. 2-Nitroimidazole (EF5) binding predicts radiation resistance in individual 9L sc tumors. Cancer Res. 1996; 56: 405-411.
- Tran LB, Bol A, Labar D, Karroum O, Bol V, Jordan B, et al. Potential role of hypoxia imaging using (18) F-FAZA PET to guide hypoxia-driven interventions (carbogen breathing or dose escalation) in radiation therapy. Radiother Oncol. 2014; 113: 204-209.
- Schutze C, Bergmann R, Bruchner K, Mosch B, Yaromina A, Zips D, et al. Effect of [(18)F]FMISO stratified dose-escalation on local control in FaDu hSCC in nude mice. Radiother Oncol. 2014; 111: 81-87.
- Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. British journal of cancer. 2015; 112: 238-250.
- Ceelen W, Smeets P, Backes W, Van Damme N, Boterberg T, Demetter P, et al. Noninvasive monitoring of radiotherapy-induced microvascular changes using dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) in a colorectal tumor model. Int J Radiat Oncol Biol Phys. 2006; 64: 1188-1196.
- Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed. 2004; 17: 240-262.
- Wilson DF, Cerniglia GJ. Oxygenation of tumors as evaluated by phosphorescence imaging. Adv Exp Med Biol. 1994; 345: 539-547.
- Horsman MR, Khalil AA, Nordsmark M, Grau C, Overgaard J. Relationship between radiobiological hypoxia and direct estimates of tumour oxygenation in a mouse tumour model. Radiother Oncol. 1993; 28: 69-71.
- Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006; 82: 699-757.
- Jerabek PA, Patrick TB, Kilbourn MR, Dischino DD, Welch MJ. Synthesis and biodistribution of 18F-labeled fluoronitroimidazoles: potential in vivo markers of hypoxic tissue. Int J Rad Appl Instrum [A]. 1986; 37: 599-605.
- Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, et al. Imaging of hypoxia in human tumors with [F-18] fluoromisonidazole. Int J Radiat Oncol Biol Phys. 1992; 22: 199-212.
- Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005; 46: 106-113.
- Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, et al. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia Radiology. 1995; 194: 795-800.
- Chitneni SK, Bida GT, Zalutsky MR, Dewhirst MW. Comparison of the Hypoxia PET Tracer (18) F-EF5 to Immuno histo chemical Marker EF5 in 3 Different Human Tumor Xenograft Models. See comment in PubMed Commons below J Nucl Med. 2014; 55: 1192-1197.
- Van Loon J, Janssen MH, Ollers M, Aerts HJ, Dubois L, Hochstenbag M, Dingemans AM. PET imaging of hypoxia using [18F] HX4: a phase I trial. See comment in PubMed Commons below Eur J Nucl Med Mol Imaging. 2010; 37: 1663-1668.
- Seelam SR, Lee JY, Lee YS, Hong MK, Kim YJ, Banka VK, et al. Development of (68) Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorganic & medicinal chemistry. 2015; 23: 7743-7750.
- Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. See comment in PubMed Commons below J Nucl Med. 1997; 38: 1155-1160.
- Prekeges JL, Rasey JS, Grunbaum Z, Krohn KH. Reduction of fluoromisonidazole, a new imaging agent for hypoxia. See comment in PubMed Commons below Biochem Pharmacol. 1991; 42: 2387-2395.
- Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007; 167: 127-145.
- Gagel B, Reinartz P, Dimartino E, Zimny M, Pinkawa M, Maneschi P, et al. pO(2) Polarography versus positron emission tomography ([(18) F] fluoromisonidazole, [(18)F]-2-fluoro-2’-deoxyglucose). An appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol. 2004; 180: 616- 622.
- Rasey JS, Nelson NJ, Chin L, Evans ML, Grunbaum Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. See comment in PubMed Commons below Radiat Res. 1990; 122: 301-308.
- Rasey JS, Casciari JJ, Hofstrand PD, Muzi M, Graham MM, Chin LK. Determining hypoxic fraction in a rat glioma by uptake of radiolabeled fluoromisonidazole. See comment in PubMed Commons below Radiat Res. 2000; 153: 84-92.
- Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, Chan K. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2008; 70: 2-13.
- Rajendran JG, Schwartz DL, O’Sullivan J, Peterson LM, Ng P, Scharnhorst J, Grierson JR. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. See comment in PubMed Commons below Clin Cancer Res. 2006; 12: 5435-5441.
- Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, Machulla HJ. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. See comment in PubMed Commons below J Nucl Med. 2005; 46: 253-260.
- Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004; 10: 2245-2252.
- Lawrentschuk N, Poon AM, Foo SS, Putra LG, Murone C, Davis ID, Bolton DM. Assessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomography. See comment in PubMed Commons below BJU Int. 2005; 96: 540-546.
- Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. See comment in PubMed Commons below J Nucl Med. 2004; 45: 1851-1859.
- Cher LM, Murone C, Lawrentschuk N, Ramdave S, Papenfuss A, Hannah A, O’Keefe GJ. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. See comment in PubMed Commons below J Nucl Med. 2006; 47: 410-418.
- Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemo radiation with or without tirapazamine: a sub study of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006; 24: 2098-2104.
- Grönroos T, Bentzen L, Marjamäki P, Murata R, Horsman MR, Keiding S, Eskola O. Comparison of the biodistribution of two hypoxia markers [18F] FETNIM and [18F] FMISO in an experimental mammary carcinoma. See comment in PubMed Commons below Eur J Nucl Med Mol Imaging. 2004; 31: 513-520.
- Dubois LJ, Lieuwes NG, Janssen MH, Peeters WJ, Windhorst AD, Walsh JC, Kolb HC. Preclinical evaluation and validation of [18F] HX4, a promising hypoxia marker for PET imaging. See comment in PubMed Commons below Proc Natl Acad Sci U S A. 2011; 108: 14620-14625.
- Zegers CM, van Elmpt W, Wierts R, Reymen B, Sharifi H, Ollers MC, et al. Hypoxia imaging with [(1)(8)F] HX4 PET in NSCLC patients: defining optimal imaging parameters. Radiother Oncol. 2013; 109: 58-64.
- Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, Dubois L. A comparative study of the hypoxia PET tracers [¹â¸F] HX4, [¹â¸F] FAZA, and [¹â¸F] FMISO in a preclinical tumor model. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2015; 91: 351-359.
- Vavere AL, Lewis JS. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. See comment in PubMed Commons below Dalton Trans. 2007; 4893-4902.
- Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis (N4-methylthiosemicarbazone). See comment in PubMed Commons below J Nucl Med. 2008; 49: 201-205.
- Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, Welch MJ. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. See comment in PubMed Commons below Eur J Nucl Med Mol Imaging. 2003; 30: 844-850.
- Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu (II)-diacetyl-bis (N4-methylthiosemicarbazone). See comment in PubMed Commons below J Nucl Med. 2006; 47: 989-998.
- O’ Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazoleand 64Cu (II)-diacetyl- bis (N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring micro PET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327- AT and FaDu rat tumor models. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2005; 61: 1493-1502.
- Colombié M, Gouard S, Frindel M, Vidal A, Chérel M, Kraeber-Bodéré F, Rousseau C . Focus on the Controversial Aspects of (64) Cu-ATSM in Tumoral Hypoxia Mapping by PET Imaging. See comment in PubMed Commons below Front Med (Lausanne). 2015; 2: 58.
- Malinen E, Søvik A, Hristov D, Bruland ØS, Olsen DR. Adapting radiotherapy to hypoxic tumours. See comment in PubMed Commons below Phys Med Biol. 2006; 51: 4903-4921.
- Toma-Dasu I, Dasu A. Quantitative hypoxia imaging for treatment planning of radiotherapy. See comment in PubMed Commons below Adv Exp Med Biol. 2014; 812: 143-148.
- Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res. 2004; 64: 1886-1892.
- Toma-Daşu I, Daşu A, Brahme A. Quantifying tumour hypoxia by PET imaging--a theoretical analysis. See comment in PubMed Commons below Adv Exp Med Biol. 2009; 645: 267-272.
- Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F] fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996; 36: 417-428.
- Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K, Kuge Y. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. See comment in PubMed Commons below J Nucl Med. 2013; 54: 201-207.
- Muzi M, Peterson LM, O’Sullivan JN, Fink JR, Rajendran JG, McLaughlin LJ, et al. 18F-Fluoromisonidazole Quantification of Hypoxia in Human Cancer Patients Using Image-Derived Blood Surrogate Tissue Reference Regions. J Nucl Med. 2015; 56: 1223-1228.
- Mönnich D, Welz S, Thorwarth D, Pfannenberg C, Reischl G, Mauz PS. Robustness of quantitative hypoxia PET image analysis for predicting local tumor control. See comment in PubMed Commons below Acta Oncol. 2015; 54: 1364-1369.
- Kirkpatrick JP, Cárdenas-Navia L, Dewhirst MW. Predicting the effect of temporal variations in PO2 on tumor radiosensitivity. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2004; 59: 822-833.
- Alper T, Howard-Flanders P. Role of oxygen in modifying the radiosensitivity of E. coli B. See comment in PubMed Commons below Nature. 1956; 178: 978-979.
- Popple RA, Ove R, Shen S. Tumor control probability for selective boosting of hypoxic sub volumes, including the effect of reoxygenation. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2002; 54: 921-927.
- Wang JZ1, Li XA, Mayr NA. Dose escalation to combat hypoxia in prostate cancer: a radiobiological study on clinical data. See comment in PubMed Commons below Br J Radiol. 2006; 79: 905-911.
- Zhou SM, Das SK, Wang Z, Sun X, Dewhirst M, Yin FF, et al. Self-consistent tumor control probability and normal tissue complication probability models based on generalized EUD. Med Phys. 2007; 34: 2807-2815.
- Tomé WA, Fowler JF. Selective boosting of tumor sub volumes. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2000; 48: 593-599.
- Chang SX, Cullip TJ, Deschesne KM. Intensity modulation delivery techniques: “step & shoot” MLC auto-sequence versus the use of a modulator. See comment in PubMed Commons below Med Phys. 2000; 27: 948-959.
- Gutiontov SI, Shin EJ, Lok B, Lee NY, Cabanillas R. Intensity-modulated radiotherapy for head and neck surgeons. See comment in PubMed Commons below Head Neck. 2015.
- Chan C, Lang S, Row bottom C, Guckenberger M, Faivre-Finn C, Committee IART. Intensity-modulated radiotherapy for lung cancer: current status and future developments. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2014; 9: 1598-1608.
- Lin Z, Mechalakos J, Nehmeh S, Schoder H, Lee N, Humm J, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008; 70: 1219-1228.
- Nehmeh SA, Lee NY, Schroder H, Squire O, Zanzonico PB, Erdi YE, Greco C . Reproducibility of intratumor distribution of (18) F-fluoromisonidazole in head and neck cancer. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2008; 70: 235-242.
- Kempf H, Bleicher M, Meyer-Hermann M. Spatio-Temporal Dynamics of Hypoxia during Radiotherapy. See comment in PubMed Commons below PLoS One. 2015; 10.
- Eschmann SM1, Paulsen F, Bedeshem C, Machulla HJ, Hehr T, Bamberg M, Bares R. Hypoxia-imaging with (18) F-Misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. See comment in PubMed Commons below Radiother Oncol. 2007; 83: 406-410.
- Fatema CN, Zhao S, Zhao Y, Yu W, Nishijima K, Yasuda K, et al. Dual tracer evaluation of dynamic changes in intratumoral hypoxic and proliferative states after radiotherapy of human head and neck cancer xenografts using radiolabeled FMISO and FLT. BMC Cancer. 2014; 14: 692.
- Bradshaw TJ, Yip S, Jallow N, Forrest LJ, Jeraj R. Spatiotemporal stability of Cu-ATSM and FLT positron emission tomography distributions during radiation therapy. Int J Radiat Oncol Biol Phys. 2014; 89: 399-405.
- Thorwarth D, Alber M. [Individualised radiotherapy on the basis of functional imaging with FMISO PET]. Zeitschrift fur medizinische Physik. 2008; 18: 43- 50.
- Søvik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS, Olsen DR. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2007; 68: 1496-1504.
- Søvik A, Malinen E, Olsen DR . Strategies for biologic image-guided dose escalation: a review. See comment in PubMed Commons below Int J Radiat Oncol Biol Phys. 2009; 73: 650-658.
- Choi W, Lee SW, Park SH, Ryu JS, Oh SJ, Im KC, et al. Planning study for available dose of hypoxic tumor volume using fluorine-18-labeled fluoromisonidazole positron emission tomography for treatment of the head and neck cancer. Radiother Oncol. 2010; 97: 176-182.
- Henriques de Figueiredo B, Zacharatou C, Galland-Girodet S, Benech J, De Clermont-Gallerande H, Lamare F, et al. Hypoxia imaging with [18F]-FMISOPET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancers. Strahlenther Onkol. 2014.
- Servagi-Vernat S1, Differding S, Sterpin E, Hanin FX, Labar D, Bol A, Lee JA . Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. See comment in PubMed Commons below Acta Oncol. 2015; 54: 1008-1016.