
Review Article
Austin J Nucl Med Radiother. 2016; 3(1): 1017.
Applications of Micro-CT in Imaging Heart Diseases of the Murine Models
Wang Y¹, Yu C¹, Gao S¹, Lin Li¹, Chen W²* and Zhu Y³*
¹Tianjin University of Traditional Chinese Medicine, China
²Department of Molecular Imaging and Nuclear Medicine, Tianjin Medical University Cancer Institute and Hospital, China
³Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, China
*Corresponding author: Yan Zhu, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China 300193
Wei Chen, Molecular Imaging & Nuclear Medicine Department, Tianjin Medical University Cancer Hospital, China
Received: March 11, 2016; Accepted: May 24, 2016; Published: May 27, 2016
Abstract
Objective: This non-systematic review discusses the feasibility and advancements on the application of micro-Computed Tomography (micro-CT) in the experimental scope of murine cardiac models.
Methods: Medline and Elsevier were searched for inclusion of relevant studies. A total of 69 articles were downloaded by using ‘micro-CT’, ‘murine’, and ‘heart’ as the keywords including synonyms like ‘mouse’, ‘rat’, ‘rodent’, and 47 of them were retained after review. No limitations in time were considered.
Results: The major application of micro-CT in murine heart research included the following disease models: Atherosclerosis (AS), Myocardial Infarction (MI), Coronary Artery Disease (CAD), Congenital Heart Defects (CHD) and Ischemia/Reperfusion (I/R). Experimental advancements in recent years of micro-CT are listed, while limitations and challenges of micro-CT are briefly discussed. Future trends of the imaging technology are also mentioned in the following part.
Conclusion: A number of years of preclinical practices have proven the feasibility and efficacy of micro-CT. However, new studies based on multimodality are still in demand to help strengthen our understanding of the mechanisms that give rise to the progression of cardiac diseases.
Keywords: Micro-CT; Small animal modeling; In vivo; Ex vivo; Heart disease
Abbreviations
Micro-CT: Micro-Computed Tomography; AS: Atherosclerosis; MI: Myocardial Infarction; CAD: Coronary Artery Disease; CHD: Congenital Heart Defects; I/R: Ischemia/Reperfusion; CNT: Carbon Nano Tube; Micro-CTA: Micro-Computed Tomography Angiography; DM: Diabetes Mellitus; ApoE: ApolipoproteinE; LF: Left Ventricle; CO: Cardiac Output; SV: Stroke Volume; MRI: Magnetic Resonance Imaging; DOB: Dobutamine; Micro-PET: Micro Positron Emission Tomography; Micro-SPECT: Micro Single- Photon Emission Computed Tomography.
Introduction
Over the past 20 years, technical advances in picture acquisition and imaging capabilities have vastly increased the quality and quantity of anatomical and physiopathologic data. Micro-Computed tomography (Micro-CT) is a relatively new modality that rapidly improves high spatial resolution imaging of subtle structures. Due to its high density resolution, relatively low cost and scanning efficiency, micro-CT imaging has been improved over the last decades and has shown its utility in many preclinical practices. Micro-CT, suitable for either ex vivo or in vivo imaging, has evolved from custom-made to commercially available scanner. The purpose of this paper is to provide an overview of applications of micro-CT on murine models with a focus on the diagnostic accuracy and preclinical value in heart disease. Experimental advancements in recent years of micro- CT are listed, while limitations and challenges of micro-CT are briefly discussed. Future trends of the imaging technology are also mentioned in the following section.
CT imaging in clinical use
CT scanner has rapidly evolved from single slice to multi-slice which started from 4-slice systems in 1998 to the latest 256-slice and even 320-slice CT systems [1]. Becker H-C [2] from reviewing the literature and clinical results concluded that cardiac CT could accurately diagnose heart disease or other sources of chest pain, markedly decrease health care expenditure, and reliably predict clinical outcomes with appropriate patient selection.
Cardiac imaging with coronary CT angiography provide indications about: (1) evaluation of coronary arteries for atherosclerosis or anomalies; (2) evaluation of noncoronary pathology including the great vessels, chambers, myocardium, valves, or pericardium; (3) evaluation of cardiac chamber function, including ejection fraction and chamber volumes; (4) evaluation of low-to-intermediate risk symptomatic patients presenting with symptoms of stable angina or acute chest pain; and (5) discordant or inconclusive stress tests [3]. In a recent study [4], it was reported that the sensitivity and specificity of 320-slice Computed Tomography Angiography (CTA) were 100% and 87% to detect significant Coronary Artery Disease (CAD) in patients with acute chest pain in the Emergency Department.
For the cardiac surgeons, the main benefits of Multi-Detector-Row CT (MDCT) lie in the combination of large scan-volume coverage, high spatial resolution, decent identification of calcifications, and the record other thoracic structures simultaneously. Preoperative applications may include the assessment of heart valves, noninvasive evaluation of large thoracic vessels, staging of cardiac tumors, and programming of minimally invasive surgical procedures. After surgery, MDCT examinations particularly facilitate an early identification of severe postoperative complications [5]. MDCT may also be used to assess coronary artery bypass graft patency and to detect transplant-related complications in heart transplant recipients at an early stage. For instance, CT is the modality of choice in patients with aortic stenosis arranged for planning of aortic valve implantation. A multicenter trial of 1038 European patients enrolled at 32 centers (SOURCE-registry) showed overall survival of 76.1% after one year [6,7]. A two-year follow-up of patients in the placement of aortic transcatheter valves (PARTNER) trial supported it as an alternative to surgery in high risk patients with the death rate of 33.9% [8].
Applications of micro-CT in murine heart
Apart from integrative murine modeling of normal physiological function, micro-CT has been successfully used for detecting diseases of bone fracture [9,10], lung fibrosis [11], nonalcoholic fatty liver disease [12] and cardiac injury [13], and understanding mechanisms of pathological conditions. This paper summarized the applications of micro-CT in murine with a focus on the heart. Calcifications, atherosclerosis plagues, shape of vessel and cardiac structure were detected with or without contrast material. Cardiac functional metrics can be computed by 4D cardiac micro-CT data sets. All of the concerned articles were listed in (Table 1) chronologically.
Authors
Publication Year
Animal Models
Objective
Contrast Agents
Type
Detombe S A, et al [22]
2008
MI mice model
Cardiac Function
Fenestra VC(ART, Qc, Canada
in Vivo
Martinez H G, et al [17]
2009
ApoE Knockout mouse
Atherosclerotic plagues
without
ex vivo
Sebastian J, et al [19]
2010
C57BL/6 mice
Cerebral, thoracic and abdominal vasculature
Imeron300(INN,Latin) and Fenestra VC
in Vivo
Badea C T, et al [25]
2011
DOB-induced cardiac stress rats
Cardiac Function
liposomal-based blood pool contrast agent
in Vivo
Pai V M, et al [18]
2012
CAD mouse model
coronary artery wall and lipid deposition
OsO4
ex vivo
Detombe S A, et al [47]
2012
C57BL6/ and BALB/c mice
enhancement-time curves of different tissues
eXIA160(Binitio Biomedical, Ottawa, Canada)
in vivo
Sangaralingham S, et al [20]
2012
Fisher rats
myocardial volume of intramyocardial and epicardial vessels
microfil(Ladd Research, Williston,USA)
ex vivo
Vandoorne K, et al [23]
2013
MI mouse model
cardiac function and angiogenesis
microfil MV120
ex vivo
Detombe S A [47]
2013
C57BL/6 mice
lung volume, lung density, left ventricular volume and ejection fraction
without
ex vivo
Kim A J, et al [21]
2013
CHD mouse model
identifying a wide spectrum of CHD
iodine contrast-enhanced agents
in vivo
Wait J M, et al [15]
2013
ApoE-null mice
calcification volume and plaque areas
without
in vivo
Le Quang K, et l [16]
2014
a mouse model of combined dyslipidemia and type 2 diabetes mellitus
calcification in the aortic valves
without
in vivo
Lee C L, et al [13]
2014
mice after partial-heart irradiation
permeability of myocardial vessels and cardiac physiology indexes
AuNp
in vivo
Burk L M, et al [24]
2015
I/R mice model
delayed contrast enhancement in the LV wall and cardiac function Omnipaque
300(GE Healthcare, Cork, Ireland) and Fenestra VC
in vivo
MI: Myocardial Infarction; apoE: ApolipoproteinE; DOB: Dobutamine; CAD: Coronary Artery Disease; CHD: Congenital Heart Defects; I/R: Ischemia Reperfusion
Table 1: The checklist of applications of cardiac micro-CT in murine.
Calcifications
Because of its enchanting characteristics such as fast switching, electronic programmability, distributed source, and multiplexing, Carbon Nano Tube (CNT) based field emission x-ray source technology has newly been investigated for diagnostic imaging applications. The feasibility for prospective-gated cardiac micro-CT imaging of freebreathing mice under their natural position was demonstrated [14]. Calcification volume and plaque areas were measured using CNTbased x-ray source in the aortic arch of ApolipoproteinE (ApoE)–null mice [15]. Last year, calcification in the aortic valves was detected in a mouse model of combined dyslipidemia and type 2 Diabetes Mellitus (DM) [16]. It demonstrated that the dysmetabolic state of type 2 DM impelled early mineralization of the aortic valve and calcified aortic valve disease pathogenesis.
Atherosclerosis plagues
When stained with a pre-commercial staining solution, excised hearts from an apoE knockout mouse showed atherosclerotic plaques in the aortic leaflet and ascending aorta [17]. Furthermore, freely available software tools exist for the visualization of natural edge boundary features of 3-dimensional tissues as well as volume quantification of atherosclerotic lesions at multiple foci to microliter accuracy. Vinay M Pai et al [18]. Demonstrated that a combination of OsO4 (osmium tetroxide) and micro-CT permitted the visualization of the coronary artery wall in intact apoE knockout mouse hearts. Additionally, since OsO4 preferentially attaches to lipids, it highlighted lipid deposition in the artery wall. This imaging protocol could potentially be a very useful implement for detecting plaques in the coronary arteries of mouse Coronary Artery Disease (CAD) models.
Morphology of vessels and heart chambers
Schambach and coworkers described a protocol for in vivo micro- CTA (micro-computed tomography angiography) in mice using both a bolus technique with a conventional contrast agent, Imeron 300 (INN, Latin) and angiography with a blood-pool contrast agent, Fenestra VC (ART, QC, Canada). The contrasts of vascular structures of brain, thorax and abdomen with these two agents were compared. From this initial experiment we learned, that using a blood-pool contrast agent the vessels were well detected [19].
To figure up the myocardial volume of intramyocardial and epicardial vessels, the isolated microfilm (Ladd Research, Williston, USA) injected into the rat hearts were harvested and prepared for scanning on a high resolution, volumetric custom build micro-CT scanner. Fischer rats of different ages separated into two groups underwent cardiac micro-CT imaging as well as echocardiography, blood pressure and fibrosis analysis. The results illustrated the reduction in normalized intramyocardial vessel volume of the aged hearts, in association with increased epicardial vessel volume, in the setting of increased Left Ventricle (LV) fibrosis and mild LV dysfunction [20].
Kim A J et al. [21] investigated the efficacy of micro-CT to screen Congenital Heart Defects (CHD) in stillborn/fetal mice. Analysis of 2105 fetal/newborn mice by iodine contrast-enhanced micro-CT showed this imaging modality was highly effective in identifying a wide spectrum of CHD. Overall, they observed an accuracy of 89.8% for diagnosing ventricular septal defects. Outflow tract anomalies were diagnosed with 97.4% accuracy. Accuracy of detecting aortic arch anomalies was 99.6%.
Cardiac function
Nowadays, global cardiac functional metrics such as Cardiac Output (CO), Stroke Volume (SV), ejection fraction, and myocardial mass as well as dynamic metrics such as wall motion can be computed by 4D cardiac micro-CT data sets.
Equipped with retrospective gating, cardiac function in sham and the infracted mice could be evaluated longitudinally. Significant differences in the systolic volumes, diastolic volumes and EF, between the sham and the Myocardial Infarction (MI) groups were detected [22].
Similarly, excised hearts filling with microfil MV120, a radioopaque silicone rubber, in the cardiac arteries were used to investigate the impact of systemic Akt1 deficiency on cardiac function and angiogenesis before and after MI. Magnetic Resonance Imaging (MRI) revealed mildly decreased baseline cardiac function in Akt1 null mice, whereas ex vivo stereomicroscopy and micro-CT revealed substantially the reduced coronary macrovasculature [23]. This longitudinal study provided clear evidence that mice with chronic loss of Akt1 exhibited improved heart function and reduced LV remodeling after experimental MI. Long-term inhibition of Akt1 might offer an alternative therapeutic strategy aimed to reduce secondary damage caused by cardiac remodeling.
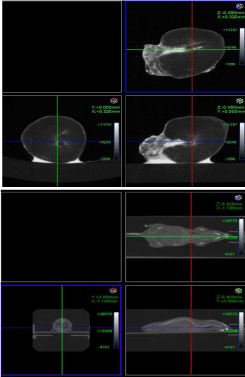
Figure 1: 3D CT imaging of mouse heart, the upper one ex vivo, the lower
one in vivo.
Choi and coworkers found that the AuNp contrast agent and delayed DE micro-CT could be utilized to non-invasively assess the change in permeability of myocardial vessels after partial-heart irradiation [13]. They also measured a number of clinically important endpoints of cardiac physiology, including the LV end-diastolic volume, end-systolic volume, SV, EF, and CO.
Burk L M, et al [24] demonstrated the ability to identify areas of myocardial infarct consistently in mice and provided functional cardiac information using a delayed contrast enhancement technique.
Drug safety
Cardiovascular safety is an important concern in contemporary drug development and a significant contributor to safety-related attrition of novel drugs in development. Micro-CT has also been used for the evaluation of drug effects, such as shown in a study on assessment of Dobutamine (DOB) induced cardiac stress in rats [25]. In order to assess normal response to DOB stress in rats, SV, EF and correlative peripheral arterial pressures associated with the significant increases in CO were measured. Accordingly, the impact of such an enabling technology can be tremendous in evaluating cardiotoxic effects of various test drugs.
Technical limitations of micro-CT
Not only micro-CT, but also several other imaging modalities have been adapted from their clinical counterparts for animal experiments, such as high-frequency ultrasound, micro Positron Emission Tomography (micro-PET), micro Single-Photon Emission Computed Tomography (micro-SPECT) and MRI. Compared to other imaging modalities, micro-CT has its technical merits and drawbacks (Table 2).
Advantages
Disadvantages
Ultrasound
measure the size of organ, volume and blood flow velocities
Inexpensive
repeatedly and dynamically inexpensive without radiation
low specificity
Micro-PET
high sensitivity
low signal to noise ratio
high specificity
expensive
security guaranteed
low spatial resolution contrast agents needed
Micro-SPECT
simultaneous anatomic
low accuracy and resolution
functional
low contrast to noise ratio
molecular imaging
contrast agents needed
MRI
no radioactive damage
long acquisition time
multi-parameter imaging and high contrast to noise
more costly
ratio and signal to noise ratio
Technically complicated
Micro-CT
high spatial resolution
high density resolution
time-saving
cost-effective
effect of radiation
Table 2: Comparison between micro-CT and other imaging modalities.
First, radiation dose associated with micro-CT methods is not negligible. X-ray exposure can be harmful since it can disrupt chemical bonds and create free radicals in the body. Typically, the whole-body radiation dose for a 3D micro-CT scan reported in the literature ranges from 0.017Gy to 0.78Gy, depending on the diagnostic demand and the contrast resolution required [26]. On the opposite, ultrasound, echocardiography and micro-PET, remaining the cornerstone for diagnosing and monitoring heart disease, are not interfered with radioactive damage [27-30]. So there has been a continuing effort to improve security guarantee of this condition with micro-CT.
Second, the low X-ray absorption of non-mineralized tissues is one of the major challenges for micro-CT imaging so that contrast agents are commonly involved to increase the lesion-to-tissue ratio. Kinds of contrast materials have emerged in need. Iodine-based, low molecular weight contrast agents designed for clinical CT imaging applications (e.g. Omnipaque from GE Healthcare, Isovue from Bracco Diagnostic) can also be used for preclinical micro-CT imaging in animals even though they clear from mouse vasculature within seconds [31]. Iodine-based, blood pool contrast materials (e.g. Fenestra from ART, eXIA from Binitio Biomedical) provide stable enhancement over the course of minutes to an hour [32]. In addition, dose of contrast agents and the way of injection have an effect on the practice concerned with soft tissues [33].
Future trends of the imaging technology
CT imaging will continue to make progress in multiple sources, multiple-slice, multi-domain and multi-function, so that the improvements in scan speed, coverage, image quality and application value could be achieved. On the other hand, multimodality cardiovascular imaging which involves combination of at least two cardiovascular imaging techniques is a certain tendency in both clinical and experimental fields. They are typically combined in a side-by-side or fusion mode in order to present functional and morphological data to better delineate heart disease, most frequently used as PET/CT and SPECT/CT [34], with more proven efficacy than the modality used separately. Furthermore, the integration of vessel anatomy and myocardial perfusion imaging is admitted to provide better diagnostic and prognostic information that could be translated into improved level of experiments [35].
Discussion
Generally speaking, micro-CT is an imaging scanner allowing the virtual reconstruction of objects with pixel size in the micrometer range. X-rays generated by the X-ray tube emit toward the sample and the detector measures the intensity of the transmitted X-rays on the opposite side. Users get different attenuated X-ray shadow images depending on the length traveled in the absorbing material, the material composition and its density (i.e. attenuation coefficient). The 2D gray images projections, also referred to as slice plans, are reconstructed using mathematical (e.g. Filtered Back Projection FBP [36]) and iterative algorithms (e.g. Algebraic Reconstruction Technique ART [37]). For example, cone-beam source uses the Feldkamp algorithm as a tomographic reconstruction algorithm [38]. Finally, the reconstructed 2D radiographs are gathered and stacked together. As a result, the complete 3D map of the sample is computed and available for further processing [39]. Reconstructing isotropic voxels allows visualization in any orientation as 2D slices or a rendered 3D volume.
As we know, the contrast properties of CT significantly depend on the X-ray energy spectrum used to measure the object. Conventional CT uses a single energy spectrum and suffers from ambiguity at times so that two different materials that share similar grayscale intensity values as in the case of bone and iodine can appear identical. Dual energy CT yields precise anatomic and functional images by using two different energy spectra that can remove this ambiguity [40]. A two-tube/detector system ensures simultaneous acquisition of two projections, thus reducing scanning time and the doses of contrast injections in studies. The additional metallic beam filters are placed between the source and the specimen, like 1-2 mm of aluminum or copper. These metallic beam filters can be used to preferentially remove low energy photons and to improve spectral separation between polychromatic scans [41]. Various dual energy micro-CT sampling strategies are feasible, such as single source sequential scanning at two different kVps, simultaneous dual source acquisition and single source with kVp switching [42].
The proposed method produces 5D volumetric images that distinguish different materials at different points in time, and can be used to segment regions containing iodinated blood and calculate cardiac function [43]. Projection interpolation and 5D bilateral filtration (three spatial dimensions + time + energy) help to reduce noise and artifacts associated with retrospective gating. With cardiac MRI as standard of reference, double-source CT was confirmed to offer the possibility to quantify left ventricular function from coronary CT angiography datasets with sufficient diagnostic accuracy, adding to the value of the modality in a comprehensive cardiac assessment [44].
Micro-CT can provide versatile, high-contrast, quantitative in vivo or ex vivo images of small animals. The radiation dose and low x-ray contrast of soft tissues are widely recognized; however, newly developed contrast agents and novel acquisition and reconstruction strategies show extraordinary potential in overcoming these limitations and challenges. We just summarize the applications of micro-CT on heart diseases of murine, but actually it can be used on a variety of animal specimens. The breadth of possible applications has been illustrated with kinds of micro-CT images of model and nonmodel animals, including volume and section images of vertebrates, insects, embryos, and other invertebrates [45]. Chinese medicine is another field that waiting for micro-CT to realize its value. Although comprehensive micro-CT protocols rapidly provide convincing result for the diagnosis of heart diseases, the appropriate usage should be balanced against the implied exposure to radiation and contrast material, therapeutically effects and associated costs.
Acknowledgement
This research was supported by grants from the National Key Basic Research Program of China, also named as 973 Project (2014CB542902) and the National Natural Science Foundation of China (81202795).
References
- Sabarudin A, Sun Z. Coronary CT angiography: Diagnostic value and clinical challenges. World J Cardiol. 2013; 5: 473-483.
- Becker HC, Johnson T. Cardiac CT for the assessment of chest pain: imaging techniques and clinical results. Eur J Radiol. 2012; 81: 3675-3679.
- Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014; 8: 342-358.
- Van Velzen JE, de Graaf FR, Kroft LJ, de Roos A, Reiber JHC, Bax JJ, et al. Performance and efficacy of 320-row computed tomography coronary angiography in patients presenting with acute chest pain: results from a clinical registry. The international journal of cardiovascular imaging. 2012; 28: 865-876.
- Herzog C, Wimmer-Greinecker G, Schwarz W, Dogan S, Moritz A, Fichtlscherer S, et al. Progress in CT imaging for the cardiac surgeon. Seminars in Thoracic and Cardiovascular Surgery. 2004; 16: 242-248.
- Lefèvre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011; 32: 148-157.
- Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011; 124: 425-433.
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012; 366: 1686-1695.
- Collins CJ, Vivanco JF, Sokn SA, Williams BO, Burgers TA, Ploeg HL. Fracture healing in mice lacking Pten in osteoblasts: a micro-computed tomography image-based analysis of the mechanical properties of the femur. Journal of biomechanics. 2015; 48: 310-317.
- Hoerth RM, Baum D, Knötel D, Prohaska S, Willie BM, Duda GN. Registering 2D and 3D imaging data of bone during healing. Connect Tissue Res. 2015; 56: 133-143.
- Choi EJ, Jin GY, Bok SM, Han YM, Lee YS, Jung MJ, et al. Serial micro-CT assessment of the therapeutic effects of rosiglitazone in a bleomycin-induced lung fibrosis mouse model. Korean journal of radiology: official journal of the Korean Radiological Society. 2014; 15: 448-455.
- Kampschulte M, Stöckl C, Langheinrich AC, Althöhn U, Bohle RM, Krombach GA. Western diet in ApoE-LDLR double-deficient mouse model of atherosclerosis leads to hepatic steatosis, fibrosis, and tumorigenesis. Lab Invest. 2014; 94: 1273-1282.
- Lee CL, Min H, Befera N, Clark D, Qi Y, Das S. Assessing cardiac injury in mice with dual energy-microCT, 4D-microCT, and microSPECT imaging after partial heart irradiation. Int J Radiat Oncol Biol Phys. 2014; 88: 686-693.
- Cao G, Burk LM, Lee YZ, Calderon-Colon X, Sultana S, Lu J, et al. Prospective-gated cardiac micro-CT imaging of free-breathing mice using carbon nanotube field emission x-ray. Medical physics. 2010; 37: 5306-5312.
- Wait JM, Tomita H, Burk LM, Lu J, Zhou OZ, Maeda N. Detection of aortic arch calcification in apolipoprotein E-null mice using carbon nanotube-based micro-CT system. J Am Heart Assoc. 2013; 2: e003358.
- Le Quang K, Bouchareb R, Lachance D, Laplante MA, El Husseini D, Boulanger MC, et al. Early development of calcific aortic valve disease and left ventricular hypertrophy in a mouse model of combined dyslipidemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014; 34: 2283-2291.
- Martinez HG, Prajapati SI, Estrada CA, Jimenez F, Quinones MP, Wu I. Images in cardiovascular medicine: Microscopic computed tomographybased virtual histology for visualization and morphometry of atherosclerosis in diabetic apolipoprotein e mutant mice. Circulation. 2009; 120: 821-822.
- Pai VM, Kozlowski M, Donahue D, Miller E, Xiao X, Chen MY. Coronary artery wall imaging in mice using osmium tetroxide and micro-Computed Tomography (micro-CT). J Anat. 2012; 220: 514-524.
- Schambach SJ, Bag S, Groden C, Schilling L, Brockmann MA. Vascular imaging in small rodents using micro-CT. Methods. 2010; 50: 26-35.
- Sangaralingham SJ, Ritman EL, McKie PM, Ichiki T, Lerman A, Scott CG, et al. Cardiac micro-computed tomography imaging of the aging coronary vasculature. Circulation Cardiovascular imaging. 2012; 5: 518-524.
- Kim AJ, Francis R, Liu X, Devine WA, Ramirez R, Anderton SJ, et al. Micro computed tomography provides high accuracy congenital heart disease diagnosis in neonatal and fetal mice. Circulation Cardiovascular imaging. 2013; 6: 551-559.
- Detombe SA, Ford NL, Xiang F, Lu X, Feng Q, Drangova M. Longitudinal follow-up of cardiac structure and functional changes in an infarct mouse model using retrospectively gated micro-computed tomography. Investigative radiology. 2008; 43: 520-529.
- Vandoorne K, Vandsburger MH, Raz T, Shalev M, Weisinger K, Biton I, et al. Chronic Akt1 deficiency attenuates adverse remodeling and enhances angiogenesis after myocardial infarction. Circulation Cardiovascular imaging. 2013; 6: 992-1000.
- Burk LM, Wang KH, Wait JM, Kang E, Willis M, Lu J. Delayed contrast enhancement imaging of a murine model for ischemia reperfusion with carbon nanotube micro-CT. PLoS One. 2015; 10: e0115607.
- Badea CT, Hedlund LW, Cook J, Berridge BR, Johnson GA. Micro-CT imaging assessment of dobutamine-induced cardiac stress in rats. J Pharmacol Toxicol Methods. 2011; 63: 24-29.
- Carlson SK, Classic KL, Bender CE, Russell SJ. Small animal absorbed radiation dose from serial micro-computed tomography imaging. Molecular imaging and biology: MIB: the official publication of the Academy of Molecular Imaging. 2007; 9: 78-82.
- Zagorchev L, Mulligan-Kehoe MJ. Molecular imaging of vessels in mouse models of disease. Eur J Radiol. 2009; 70: 305-311.
- Kaushik S, Miller TT, Nazarian LN, Foster WC. Spectral Doppler sonography of musculoskeletal soft tissue masses. Journal of ultrasound in medicine: official journal of the American Institute of Ultrasound in Medicine. 2003; 22:1333-1336.
- Mahía-Casado P, García-Orta R, Gómez de Diego JJ, Barba-Cosials J, Rodríguez-Palomares JF, Aguadé-Bruix S. Update on Cardiac Imaging Techniques 2014. Revista Española de Cardiología (English Edition). 2015; 68: 129-135.
- Zhao Z, Yu Q, Mou T, Liu C, Yang W, Fang W, et al. Highly efficient onepot labeling of new phosphonium cations with fluorine-18 as potential PET agents for myocardial perfusion imaging. Molecular pharmaceutics. 2014; 11: 3823-3831.
- de Lin M, Ning L, Badea CT, Mistry NN, Qi Y, Johnson GA. A high-precision contrast injector for small animal x-ray digital subtraction angiography. IEEE Trans Biomed Eng. 2008; 55: 1082-1091.
- Ashton JR, Befera N, Clark D, Qi Y, Mao L, Rockman HA, et al. Anatomical and functional imaging of myocardial infarction in mice using micro-CT and eXIA 160 contrast agent. Contrast media & molecular imaging. 2014; 9: 161- 168.
- Socher M, Kuntz J, Sawall S, Bartling S, Kachelrieß M . The retrobulbar sinus is superior to the lateral tail vein for the injection of contrast media in small animal cardiac imaging. Lab Anim. 2014; 48: 105-113.
- Prior JO, Farhad H, Muller O. Multimodality Imaging in Ischemic Cardiomyopathy. Curr Cardiovasc Imaging Rep. 2014; 7: 9285.
- Blankstein R, Di Carli MF. Integration of coronary anatomy and myocardial perfusion imaging. Nature reviews Cardiology. 2010; 7: 226-236.
- Kita A, Onoguchi M Fau - Sugimoto K, Sugimoto K Fau - Tsuchida T, Tsuchida T Fau - Toi A, Toi A Fau - Kishimoto T, Kishimoto T Fau - Shimada M, et al. Development of Simple Processing for Deleting Undershooting Artifact Using the FBP Method horizontal line Evaluation of Simulation Data horizontal line.
- Fu J, Hu X, Velroyen A, Bech M, Jiang M, Pfeiffer F. 3D algebraic iterative reconstruction for cone-beam x-ray differential phase-contrast computed tomography. PLoS One. 2015; 10: e0117502.
- Wang B, Liu H, Zhao S, Wang G. Feldkamp-type image reconstruction from equiangular data. J Xray Sci Technol. 2001; 9: 113-120.
- Hindelang F, Zurbach R, Roggo Y. Micro Computer Tomography for medical device and pharmaceutical packaging analysis. Journal of pharmaceutical and biomedical analysis. 2015; 108: 38-48.
- Furlow B. Dual-energy computed tomography. Radiol Technol. 2015; 86: 301ct-321ct.
- Guo X, Johnston SM, Qi Y, Johnson GA, Badea CT. 4D micro-CT using fast prospective gating. Phys Med Biol. 2012; 57: 257-271.
- Ashton JR, Clark DP, Moding EJ, Ghaghada K, Kirsch DG, West JL, et al. Dual-Energy Micro-CT Functional Imaging of Primary Lung Cancer in Mice Using Gold and Iodine Nanoparticle Contrast Agents: A Validation Study. PloS one. 2014; 9: e88129.
- Johnston SM, Johnson GA, Badea CT. Temporal and spectral imaging with micro-CT. Med Phys. 2012; 39: 4943-4958.
- Busch S, Johnson TR, Wintersperger BJ, Minaifar N, Bhargava A, Rist C, et al. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. European radiology. 2008; 18: 570-575.
- Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC physiology. 2009; 9: 11.
- Detombe SA, Dunmore-Buyze J, Drangova M. Evaluation of eXIA 160 cardiac-related enhancement in C57BL/6 and BALB/c mice using micro-CT. Contrast media & molecular imaging. 2012; 7: 240-246.
- Detombe SA, Dunmore-Buyze J, Petrov IE, Drangova M. X-ray dose delivered during a longitudinal micro-CT study has no adverse effect on cardiac and pulmonary tissue in C57BL/6 mice. Acta radiologica (Stockholm, Sweden: 1987). 2013; 54: 435-441.