
Research Article
Austin J Nurs Health Care. 2014;1(1): 1002.
Acute or Reactivated Toxoplasmosis During Pregnancy, Its Impact on Birth Outcomes and the Associated Costs of Inpatient Care in the United States, 2001-2009
Mogos MF1*, Salemi JL2, de la Cruz CZ3, Groer ME4, Sultan DH5 and Salihu HM6
1Department of Community and Health Sciences, Indiana University, USA
2Department of Epidemiology and Biostatistics, University of South Florida, USA
3Department of Community and Family Health, University of South Florida, USA
4College of Nursing, University of South Florida, USA
5Department of Health Policy and Management, University of South Florida, USA
6Department of Obstetrics and Gynecology, University of South Florida, USA
*Corresponding author: Mogos MF, Department of Community and Health Sciences, School of Nursing, Indiana University, 1111 Middle Dr, Indianapolis, IN
Received: July 08, 2014; Accepted: Aug 01, 2014; Published: Aug 04, 2014
Abstract
Objective: To describe prevalence of acute or reactivated toxoplasmosis during pregnancy (ARTP) in the United States (US) and its association with maternal-fetal outcomes.
Methods: The authors conducted a cross-sectional analysis of a national sample of pregnancy-related hospital discharges using 2001-2009 annual data from the largest publicly-available National Inpatient Sample database in the US (N=42,468,049). Maternal toxoplasmosis and clinical outcomes were identified using International Classification of Diseases, 9th Edition, Clinical Modification diagnosis codes. We described the annual prevalence of ARTP and used survey logistic regression to evaluate the associations between ARTP and adverse pregnancy outcomes. The cost of inpatient care for pregnant women with ARTP was compared with inpatient care cost for those without ARTP.
Results: The national prevalence of ARTP was 2 per 100,000 pregnancyrelated discharges. Odds of a prolonged hospital stay quadrupled among ARTP cases (AOR=4.59, 95% CI: [2.81- 7.48]). Women with ARTP also had three times higher odds of having and infant with poor fetal growth (AOR= 3.41, 95% CI: [1.71-6.77]) and stillbirth (AOR= 3.41, 95% CI: [1.23-9.49]). The mean medical care cost for women with ARTP was $6,686, compared to $4,347 for women without ARTP. The excess cost associated with ARTP over the study period was $1,939,031.
Conclusion: Toxoplasmosis during pregnancy is associated with adverse maternal-fetal outcomes and increased cost of maternal inpatient care.
Keywords: Toxoplasmosis; Pregnancy; Birth outcomes; Cost
Abbreviations
AF: Adjustment Factor; APC: Annual Percent Change; ARTP: Acute or Reactivated Toxoplasmosis during Pregnancy; HCUP: Healthcare Cost and Utilization Project; AOR: Adjusted Odds Ratio; CCR: Cost-to-Charge Ratio; CMS: Center for Medicaid Services (CMS); HIV: Human Immunodeficiency Syndrome; ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; LOS: Length of Stay; NHANES: National Health and Nutrition Examination Survey; NIS: National Inpatient Sample; OR: Odds Ratio; US: United States
Introduction
Toxoplasmosis, caused by the protozoan Toxoplasma gondii, continues to be among the most common parasitic infections that affect humans. Although infection rates and seroprevalence vary considerably around the world, toxoplasmosis is a significant and costly global public health problem [1]. In the United States (U.S.), it is the third leading infectious cause of foodborne death (after salmonellosis and listeriosis) [2-6] carrying with it a projected annual cost of $2.35 billion [7]. Among the estimated 750 deaths attributed to toxoplasmosis each year in the US, it is believed that 50% are caused by eating meat contaminated with T.gondii[8]. In addition to foodborne transmission, zoonotic transmission can occur from infected cats who shed the parasite in their feces and contaminate litter boxes and/or soil where they defecate [2,9].
In the U.S., 15% of women of childbearing age (15 - 44 years) are infected with T.gondii, and there are about 400 to 4000 cases of congenital infection of T.gondii every year [10,11]. Toxoplasmosis is not a reportable disease in the US and the above report on prevalence is based on data extracted from regional studies. Vertical transmission occurs when prior infection is reactivated by a compromised immune system or when the infection occurs during the periconception or gestational periods [10-12]. Although analysis of the National Health and Nutrition Examination Survey (NHANES) has generated estimates of toxoplasmosis in the U.S. among women of childbearing age [5], national epidemiological data on toxoplasmosis among pregnant women and its impact on maternal-fetal outcomes are still lacking. This study utilizes a large, multi-year, nationally representative dataset in the US to investigate the prevalence of toxoplasmosis among pregnancy-related hospital discharges, estimate its association with adverse pregnancy outcomes, and assess its impact on the direct costs of medical care of infected pregnant women.
Materials and Methods
The authors conducted a cross-sectional analysis of pregnancyrelated hospital discharges using 2001-2009 annual data from the Nationwide Inpatient Sample (NIS), the largest all-payer, publicly-available inpatient database in the U.S [13]. Each year, the Healthcare Cost and Utilization Project (HCUP) stratifies all nonfederal community hospitals from participating states based on the American Hospital Association classification into groups based on five major hospital characteristics: rural/urban location, number of beds, geographic region, teaching status, and ownership. Within each stratum, a 20% sample of hospitals is drawn using systematic random sampling, and all inpatient discharges from selected hospitals are included. The final database from HCUP includes hospital stratum identifiers and discharge-level sampling weights to facilitate generation of national prevalence estimates that take into account the complex sampling design of the NIS.
To identify hospital stays for women who were pregnant or gave birth, we used an HCUP-created variable, NEOMAT, designed to classify hospitalizations as maternal and/or neonatal, based on the presence of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes [14]. Each hospital discharge record contains ICD-9-CM codes for a patient’s principal diagnosis and up to 14 secondary diagnoses. Beginning in 2009, the NIS included up to 24 secondary diagnosis fields. A detailed list of the specific diagnosis and procedure codes used to identify pregnancy/birth-related records was previously published [15]. Among pregnancy-related discharges, we identified women with toxoplasmosis using the 130.0-130.9 range of ICD-9-CM codes. Maternal co-morbidities and fetal outcomes including early onset delivery, poor fetal growth, and stillbirth were also identified using ICD-9-CM codes (Table 1).
Condition
International Classification of Diseases, 9th Edition, Diagnosis Code
Exposure
Toxoplasmosis
130x
Clinical comorbidities
Obesity
278.00, 278.01, 278.03, 649.1x, V85.3x, V85.4x, V85.54, 793.91
Pre-pregnancy hypertension
401x, 402x, 403x, 404x, 405x, 642.0x, 642.1x, 642.2x, 642.7x
Pre-pregnancy diabetes
249x, 250x, 648.0x
Chronic renal disease
581x, 582x, 583x, 585x, 587x, 646.2x
Coronary heart disease
410x, 411x, 412x, 413x, 414x, 429.2
Disorders of lipid metabolism
(e.g., hyperlipidemia)
272x
Perinatal history
Anemia
280x, 281x, 282x, 283x, 284x, 285x, 648.2x
Previous Cesarean section
654.2x
Multiple gestation/birth
651x, V27.2, V27.3, V27.4, V27.5, V27.6, V27.7
Eclampsia
642.6x
Pre-ecalmpsia
642.4x, 642.5x
Placenta abruption
641.1x
Placenta accreta
667.0x
Placenta previa
641.0x. 641.1x
Behavioral history
Tobacco use
305.1, 649.0x, 989.84
Alcohol use
291x, 303x, 305.0x, 425.5, 760.71, V11.3
Drug use
292.0x, 292.1x, 292.2x, 292.8x, 304x, 305.2x, 305.3x, 305.4x, 305.5x, 305.6x, 305.7x, 305.9x, 648.3x, 655.5x, 760.72, 779.5, 760.75, 965.00, 965.02, E935.1, E850.1
Fetal outcomes
Early onset delivery/preterm birth
644.2x
Stillbirth
656.4x, V27.1, V27.3, V27.4, V27.6, V27.7
Poor fetal growth
656.5x
The code suffix “x” represents all possible codes that follow the stated code prefix.
Table 1: List of International Classification of Diseases, Ninth Edition, Clinical Modification codes used to identify selected clinical and behavioral conditions.
The number of days spent in hospital was assessed as one of our outcomes. A prolonged hospitalization in this study was defined as a length of stay (LOS) that is equal to or exceeded the 95th percentile based on the distribution of LOS among all pregnancy-related discharges. In our sample 95% of all pregnancy-related discharges had less than 5 days of hospital stay. Hence, we defined prolonged LOS as a hospital stay for five or more days.
Maternal age in years was grouped into five categories: <20, 20- 24, 25-29, 30-34, and =35. In the NIS dataset, maternal race-ethnicity was first determined by self-reported ethnicity (Hispanic or non- Hispanic), with the non-Hispanic (NH) group further subdivided by race (white, black, or other). Median household income was estimated using the documented zip code of residence, and was then ranked into quartiles by HCUP. Primary payers for each hospital stay were classified into one of the following three groups: government (Medicare/Medicaid), private (commercial carriers and private HMOs and PPOs), and other sources (including self-pay and no charge). This study, also assessed the distribution of ARTP by several hospital characteristics including teaching status (teaching, in which the ratio of full-time equivalent interns and residents to non-nursing home beds is = 0.25, vs. non-teaching), urban-rural location, and U.S. census region (Northeast, Midwest, South, or West).
To compare the costs of inpatient care among pregnancy-related discharges with and without toxoplasmosis infection, we first had to convert reported charges to a refined cost estimate. While charges represent what a hospital bills for services, they do not reflect the actual cost of services rendered. Moreover, the markup from what it costs a hospital to provide its services to what it charges varies significantly across hospitals, among different departments within the same hospital, and over time [16]. Therefore, to minimize the impact of variation in cost markup, and to more accurately estimate actual resource consumption during medical care, we converted hospital charges to cost estimates using two steps [17]. First, the total charges reported in the discharge record were multiplied by a hospital-specific cost-to-charge ratio (CCR). The CCRs were calculated by HCUP using hospital accounting reports from the Center for Medicaid Services (CMS) [18]. Second, reported charges were multiplied by an HCUP-generated “adjustment factor” (AF) that attempts to account for interdepartmental variations in markup within each hospital [19]. The formula we used to calculate the estimated cost of care for each pregnancy-related discharge record is provided below:
Total cost = total charges * hospital-specific CCR * AF
Descriptive statistics were used to describe the national prevalence of ARTP among pregnancy-related discharges in the US. Distribution of socio-demographic, behavioral, and perinatal factors, hospital characteristics, and the rate of selected maternal-fetal outcomes by ARTP status were analyzed. To estimate the overall trends in ARTP, the rate of toxoplasmosis during the study period was assessed using joinpoint regression [5]. Joinpoint regression is a statistical method used to describe when there are statistically significant changes in temporal trends (increase or decrease), and to describe each trend using a model-estimated annual percent change (APC) [20].
Survey logistic regression modeling was used to calculate odds ratios (OR) and 95% confidence intervals (CI) for the association between ARTP and each outcome. For each association of interest, we constructed a crude (unadjusted) model and two multivariable (adjusted) models. Covariates were identified through a review of the literature and findings of the bivariate analyses. In the first multivariable model, we controlled for all variables listed in Table 2, and composite variable for clinical and pregnancy related morbidities (Table 3). In the second multivariable model, we also controlled for maternal human immunodeficiency virus (HIV) infection status, a strong co-morbidity, to isolate the independent effect of toxoplasmosis.
Characteristic
Na
ARTP
(%)
No ARTP
(%)
OR
(95% CI)
AORb
(95% CI)
Maternal age (years)
< 20
20 - 24
25 - 29
30 - 34
4,524,118
10,565,129
11,438,507
9,774,772
14.21
21.53
28.71
21.82
10.65
24.88
26.93
23.02
1.25 (0.71-2.22)
0.81 (0.51-1.29)
Reference
0.89 (0.56-1.42)
1.02 (0.55-1.90)
0.70 (0.43-1.15)
Reference
0.93(0.58-1.49)
Maternal race
White
Black
Hispanic
Other
Missing/Unknown
16,410,639
4,466,360
7,687,396
3,262,295
10,641,359
13.15
16.90
23.07
8.12
16.76
38.64
10.52
18.10
7.68
25.06
Reference
1.77 (0.99-3.14)
1.40 (0.90-2.19)
1.16 (0.62-2.19)
0.74 (0.45-1.20)
Reference
1.13 (0.67-1.90)
1.04 (0.65-1.66)
0.93 (0.49-1.76)
0.72 (0.44-1.15)
Drug use
No
Yes
Tobacco use
No
Yes
41,896,891
571,159
40,726,594
1,741,455
96.48
3.52
96.27
3.73
98.66
1.34
95.90
4.10
Reference
2.68 (0.85-8.43)
Reference
0.91 (0.40 - 2.06)
Reference
2.09(0.63-6.94)
Reference
0.73 (0.28-1.88)
Hospital region
Northeast
Midwest
South
West
7,096,795
9,100,601
15,976,124
10,294,529
28.26
17.05
37.72
16.97
16.71
21.43
37.62
24.24
2.42 (1.41-4.14)
1.14 (0.63-2.05)
1.43 (0.81-2.52)
Reference
1.97 (1.07-3.63)
1.28 (0.67-2.44)
1.24 (0.71-2.18)
Reference
Hospital location
Rural
Urban
5050653
37308470
7.87
92.13
11.92
88.08
Reference
1.58 (0.89-2.82)
Reference
1.23 (0.66-2.29)
Hospital teaching
Non-teaching
Teaching
22,531,984
19,827,139
35.10
64.90
53.19
46.81
Reference
2.10 (1.45-3.04)
Reference
1.79 (1.18-2.69)
Bed number
Small
Medium
Large
4,616,511
11,058,228
26,684,383
6.56
20.23
73.20
10.90
26.11
63.00
0.78 (0.40-1.50)
Reference
1.50 (0.99-2.28)
0.80 (0.41-1.56)
Reference
1.59(1.05-2.40)
Household income
Lowest quartile
2nd quartile
3rd quartile
Highest quartile
Missing/Unknown
11,316,662
10,600,270
10,120,037
9,651,594
779,486
38.82
16.98
18.45
22.85
2.90
26.65
24.96
23.83
22.73
1.84
1.45 (0.90-2.33)
0.68 (0.40-1.14)
0.77 (0.46-1.29)
Reference
1.57 (0.53-4.69)
1.09 (0.68-1.75)
0.62 (0.36-1.05)
0.74 (0.44-1.23)
Reference
1.04 (0.33-3.22)
Primary payer
Medicare/Medicaid
Private
Otherc
17,580,462
22,024,851
2,862,736
55.65
34.58
9.78
41.40
51.86
6.74
2.02 (1.43-2.84)
Reference
2.18 (1.15-4.10)
2.11 (1.44-3.08)
Reference
2.17 (1.19-3.94)
OR=odds ratio, AOR=adjusted odds ratio, CI=confidence interval
ARTP= acute or reactivated toxoplasmosis during pregnancy
HCUP= Healthcare Cost and Utilization Project, NIS= National Inpatient Sample
aWeighted to estimate national frequency; sum of all groups may not add up to the total due to missing data
bAdjusted for year of discharge and all of the other variables listed in this table
cIncludes self-pay, no charge, and other payers
Table 2: Distribution of socio-demographic, perinatal, behavioral, and hospital characteristics by ARTP status, HCUP-NIS, 2001-2009 (n= 42, 468,049).
Outcomes
Ratea
OR (95% CI)
TOXO
No TOXO
Model 1b
Model 2c
Model 3d
eHospital stay = 5 days
131.3
30.4
4.87 (2.82-8.41)
4.61(2.86-7.45)
4.59 (2.81-7.48)
Early onset delivery
101.2
66.7
1.58 (0.96-2.60)
1.47 (0.85-2.53)
1.50 (0.87-2.57)
Poor fetal growth
52.6
16.6
3.36 (1.72-6.55)
3.22 (1.61-6.44)
3.41 (1.71-6.77)
Stillbirth
23.6
6.2
3.92 (1.50-10.24)
3.31 (1.19-9.23)
3.41 (1.23-9.49)
TOXO=Toxoplasmosis, OR=odds ratio, CI=confidence interval, ARTP= acute or activated toxoplasmosis during pregnancy
HCUP= Healthcare Cost and Utilization Project, NIS= National Inpatient Sample
aPer 1,000 pregnancy-related discharges
bUnadjusted model with the presence of the condition as the outcome, Toxoplasmosis status as the exposure (“No Toxoplasmosis” is the reference group)
cModel 1 + adjustment for maternal age, race/ethnicity, household income, multiple birth, tobacco, alcohol, and drug use, primary payer, rural/urban status, composite variable for clinical morbidities (clinically diagnosed obesity, hypertension, diabestes mellitus, chronic renal diseases, coronary heart disease, hyperlipidemia), and composite variable for pregnancy related morbidities (Anemia, eclampsia, pre-eclampsia, placenta abraption, placenta accrete, and placenta previa)
dModel 2 + additional adjustment for maternal HIV infection status
eAdditional adjustment for cesarean section and dispositional status
Table 3: Outcome rates, adjusted odds ratios, and 95% confidence intervals for the associations between ARTP and selected outcomes, HCUP-NIS, 2001-2009.
To estimate the impact of ARTP on the costs of inpatient care, the mean maternal hospitalization costs between pregnancyrelated discharges with and without a toxoplasmosis diagnosis were compared. Since the cost data were positively skewed, cost was modeled using a multivariable generalized linear model with a gamma distribution and a natural log link [21]. Statistical analyses were performed with SAS software, version 9.3 (SAS Institute, Inc., Cary, MC), Stata statistical software, release 11 (StataCorp LP, College Station, TX), and the Joinpoint Regression Program, version 4.0.1 [22]. This study is considered exempt from institutional review board approval (category 4) by the University of South Florida because of the de-identified nature of the data.
Results
During the study period (2001-2009), there were 42,468,049 pregnancy-related hospital discharges, 829 of which had a diagnosis of toxoplasmosis, for a prevalence rate of 2.0 per 100,000 discharges (95% CI: 1.6 - 2.3). The rate of ARTP demonstrated a slight and inconsistent fluctuation during the study period with the highest rate in 2007 (3.6 per 100,000) and the lowest in 2002 (1.1 per 100,000). There was a non-statistically significant 4.6% annual increase between 2001 and 2009 in the number of pregnancy related discharges with the diagnosis of toxoplasmosis (Figure 1).
APC= Annual percent change, ARTP= acute or activated toxoplasmosis during pregnancy X-axis: study year Y-axis: prevalence of toxoplasmosis per 100,000 pregnancy related-discharges.
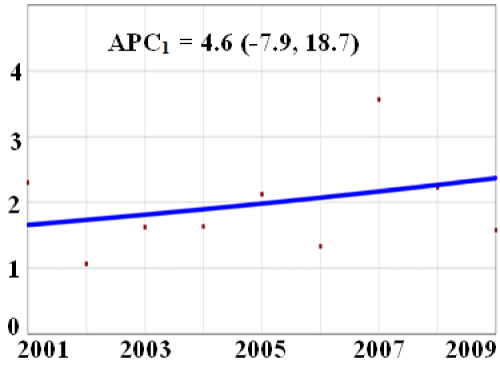
Figure 1: Trend in rates of toxoplasmosis among women with ARTP, HCUPNIS, 2001-2009.
APC= Annual percent change, ARTP= acute or activated toxoplasmosis during pregnancy X-axis: study year Y-axis: prevalence of toxoplasmosis per 100,000 pregnancy related-discharges.
Table 2 presents the distribution of maternal socio-demographic, behavioral, perinatal, and hospital characteristics by ARTP status. Women with ARTP were more likely to be on government insurance or self-pay, and be receiving care at a teaching hospital. There were no statistically significant differences in the distribution of age, race, drug use, tobacco use, and household income status across the two groups. When compared with women without ARTP, those with ARTP were more likely to be diagnosed with HIV (AOR=132, 95% CI: [75.54-230.78]), after controlling for all variables in Table 2.
In this study, ARTP was associated with diverse maternal-fetal morbidities (Table 3). Women with ARTP were over four times more likely to have a prolonged LOS (AOR= 4.59, 95% CI: [2.81-7.48]), even after controlling for other variables that potentially could impact length of hospital stay. Furthermore, ARTP was associated with over a three-fold higher risk of poor fetal growth (AOR= 3.41, 95% CI: [1.71-6.77]) and stillbirth (AOR= 3.41, 95% CI: [1.23-9.49]) in the final adjusted model.
The increased likelihood of adverse pregnancy outcomes among women with ARTP translated into higher direct inpatient medical costs. The mean maternal cost of a hospitalization with ARTP was $6,686 (95% CI: $4,995–$8,377), compared to $4,347 (95% CI: $4,190–$4,504) for pregnancy related hospitalizations without the diagnosis of acute or reactivated toxoplasmosis. Even after adjusting for maternal age, race, insurance status, and household income, the estimated difference in maternal cost, per hospitalization, was $2,339 (p =0.006). With an estimated 829 ARTP cases during the study period, the excess direct inpatient medical cost was $1,939,031.
Discussion
Although there is limited information on the rate of toxoplasmosis diagnosis among pregnant women in the U.S., estimates based on three previous regional studies projected that 400-4,000 cases of toxoplasmosis are diagnosed each year in the U.S [8]. Toxoplasmosis infection rates are lower in areas of high altitude, areas that are arid, and areas characterized by cycles of freezing and defrosting [23,24]. Consistent with this phenomenon, we observed a two-fold increased odds of Toxoplasmosis during pregnancy in the northeast. In our study, women infected with HIV were 132 times more likely to be diagnosed with toxoplasmosis than those without HIV infection. Previous studies have reported higher prevalence of toxoplasmosis (10% more) among HIV infected individuals [25,26]. This could be partly due to more targeted screening for toxoplasmosis among HIV infected pregnant women than those without HIV infection.
Recently, a study looked at the prevalence of toxoplasmosisrelated hospitalization and its co-occurrence with HIV infection among all hospital discharges in the NIS database and reported a downward trend in HIV-related toxoplasmosis from 1994 to 2002 and an upward trend in non-HIV-associated toxoplasmosis hospitalizations from 2002 to 2008 [5]. Our study expands the existing knowledge by looking at the prevalence of toxoplasmosis specifically among pregnancy-related hospitalizations and investigating its association with maternal-fetal birth outcomes. Toxoplasmosis during pregnancy is a rare clinical condition. However, it needs critical attention because it is most likely a re-activation of chronic infection or a primary acute infection and both are known risk factors for increased trans-placental transmission that can cause fetal lose or major damage to fetal health [6]. Infants born to women infected with toxoplasmosis during the first trimester are at an increased risk of congenital toxoplasmosis. This risk is even higher when the infection occurs during third trimester. However, those who acquire the infection during the first trimester are more likely to develop a severe form of the disease [23]. Therefore, proper screening for toxoplasmosis during initial antenatal care visit should be considered for women with history suggestive of potential infection with T.gondii [6], for example those who imigrated from countries where toxoplasmois infection is more prevalent. A recent study from Italy reported higher prevalence of toxoplasmosis among immigrant women from Africa, Asia, Eastern Europe, and South America [27].
Pregnant women with toxoplasmosis were over three times more likely to experience poor fetal growth and stillbirth when compared to women without the disease, even after controlling for variables that could impact outcomes under consideration. These associations remain significant even after adjusting for HIV status. This study found that ARTP was associated with a significantly higher likelihood of a prolonged hospital stay and a higher cost of medical care. These findings are likely due to a higher prevalence of HIV among women with ARTP. It is important to note that our economic analyses were from a third party payer perspective as the NIS data only contain charges from specific revenue-generating centers that are related to the institutional portion of the stay. As such, it is likely that we underestimated the costs associated with toxoplasmosis.
The results of this study should be considered in light of some limitations. First, the identification of most conditions relied exclusively on ICD-9-CM codes. These administrative data are subject to errors in coding, which increase false positive and false negative diagnoses. One exception was the use of LOS as a proxy for maternal morbidity. Recent shortenings in LOS are due to cost-cutting measures by insurance companies and managed care companies [28,29]. Given that insurance companies are not mandated to cover prolonged LOS, women who have prolonged LOS are highly likely to have suffered serious complications to justify coverage of their stay [30]. Research studies have used postpartum maternal LOS as a proxy for severity of maternal complications [30,31]. Fortney and Smith (1999) assert that LOS is a good proxy, and one study demonstrated the validity of severity of complications as a main predictive factor for the LOS for California maternity patients [32]. Even diagnostic codes cannot differentiate degrees of severity of maternal complications. Maternal morbidity is exceedingly difficult to measure with the data currently available, and thus, using LOS is an excellent proxy [32,33].
A second limitation is that maternal race-ethnicity is not reported consistently across states that provide data to the NIS. In fact, 25% of the hospitalizations in this analysis were missing race information. Third, the nature of the publicly available NIS datasets does not permit linkage of maternal delivery and infant birth hospitalizations. Therefore, although we were able to investigate the association between toxoplasmosis diagnosis during pregnancy and early onset delivery, poor fetal growth, and stillbirth, we could not assess birth-related events in the infant’s birth record. Due to the lack of a unique patient identifier, we were unable to link pregnancy-related hospitalizations for the same woman over time. Thus, by including all pregnancyrelated hospitalizations, we may have counted the same woman more than once. Despite these limitations, the large scale and nationally representative nature of the NIS data presents an opportunity to generate national prevalence estimates of toxoplasmosis during pregnancy. Future research of pregnancy-related toxoplasmosis should focus on more downstream infant outcomes, as well as on interventions that prevent both maternal toxoplasmosis and HIV infection during pregnancy, and transmission to the neonate.
References
- Robert-Gangneux F. It is not only the cat that did it: how to prevent and treat congenital toxoplasmosis. The Journal of infection. 2014; 68: S125-133.
- Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. See comment in PubMed Commons below Int J Parasitol. 2008; 38: 1257-1278.
- Jones JL, Holland GN. Annual burden of ocular toxoplasmosis in the US. See comment in PubMed Commons below Am J Trop Med Hyg. 2010; 82: 464-465.
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. See comment in PubMed Commons below Obstet Gynecol Surv. 2001; 56: 296-305.
- Jones JL, Roberts JM. Toxoplasmosis hospitalizations in the United States, 2008, and trends, 1993-2008. See comment in PubMed Commons below Clin Infect Dis. 2012; 54: e58-61.
- Paquet C, Yudin MH. Toxoplasmosis in pregnancy: prevention, screening, and treatment. See comment in PubMed Commons below J Obstet Gynaecol Can. 2013; 35: 78-79.
- Batz MB, Hoffmann S, Morris JG Jr. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. See comment in PubMed Commons below J Food Prot. 2012; 75: 1278-1291.
- Lopez A, Dietz VJ, Wilson M, Navin TR, Jones JL. Preventing congenital toxoplasmosis. See comment in PubMed Commons below MMWR Recomm Rep. 2000; 49: 59-68.
- Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. See comment in PubMed Commons below BMJ. 2000; 321: 142-147.
- Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. See comment in PubMed Commons below Clin Infect Dis. 2008; 47: 554-566.
- Remington JS, McLeod R, Thulliez PGD. Toxoplasmosis. In: Infectious Disease of the Fetus and Newborn Infant. 6th. Remington J, Klein G, Wilson CCB, editors. 6th ed. Philadelphia: W.B. Saunders. 2010; 947–1091.
- Stillwaggon E, Carrier CS, Sautter M, McLeod R. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. See comment in PubMed Commons below PLoS Negl Trop Dis. 2011; 5: e1333.
- Rockman CB, Garg K, Jacobowitz GR, Berger JS, Mussa FF, Cayne NS, et al. Outcome of carotid artery interventions among female patients, 2004 to 2005. See comment in PubMed Commons below J Vasc Surg. 2011; 53: 1457-1464.
- Authors Merrill C, Owens PL. Reasons for Being Admitted to the Hospital through the Emergency Department for Children and Adolescents, 2004: Statistical Brief #33. See comment in PubMed Commons below Reasons for Being Admitted to the Hospital through the Emergency Department for Children and Adolescents, 2004: Statistical Brief #33. ; .
- HCUP. HCUP quality control procedures 2008.
- Salemi JL, Comins MM, Chandler K, Mogos MF, Salihu HM. A practical approach for calculating reliable cost estimates from observational data: application to cost analyses in maternal and child health. See comment in PubMed Commons below Appl Health Econ Health Policy. 2013; 11: 343-357.
- Finkler SA. The distinction between cost and charges. See comment in PubMed Commons below Ann Intern Med. 1982; 96: 102-109.
- HCUP. Cost-to-Charge Ration Files (CCR). Healthcare Cost and Utilization Project (HCUP) 2001-2009 Rockville. 2013.
- Sun Y, Friedman B. Tools for more accurate inpatient cost estimates with HCUP databases, 2009. Errata added October 25, 2012. HCUP methods series report # 2011-04. Rockville, MD: U.S. Agency for Healthcare Research and Quality, 2012 Contract No.: May 10, 2013.
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. See comment in PubMed Commons below Stat Med. 2000; 19: 335-351.
- Nixon RM, Thompson SG. Parametric modelling of cost data in medical studies. See comment in PubMed Commons below Stat Med. 2004; 23: 1311-1331.
- NCI. Joinpoint Regression Program, Version 4.0.1 - January 2013; Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program National Cancer Institute. 2013.
- Feldman DM, Timms D, Borgida AF. Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy. See comment in PubMed Commons below Clin Lab Med. 2010; 30: 709-720.
- Saleh MM, AL-Shamiri AH, Qaed AA. Seroprevalence and incidence of Toxoplasma gondii among apparently healthy and visually or hearing disabled children in Taiz City, Yemen. See comment in PubMed Commons below Korean J Parasitol. 2010; 48: 71-73.
- Nissapatorn V, Kamarulzaman A, Init I, Tan LH, Rohela M, Norliza A, et al. Seroepidemiology of toxoplasmosis among HIV-infected patients and healthy blood donors. See comment in PubMed Commons below Med J Malaysia. 2002; 57: 304-310.
- Shimelis T, Tebeje M, Tadesse E, Tegbaru B, Terefe A. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia: A comparative cross-sectional study. See comment in PubMed Commons below BMC Res Notes. 2009; 2: 213.
- Capretti MG, De Angelis M, Tridapalli E, Orlandi A, Marangoni A, Moroni A, et al. Toxoplasmosis in pregnancy in an area with low seroprevalence: is prenatal screening still worthwhile? See comment in PubMed Commons below Pediatr Infect Dis J. 2014; 33: 5-10.
- Eaton AP. Early postpartum discharge: recommendations from a preliminary report to Congress. See comment in PubMed Commons below Pediatrics. 2001; 107: 400-403.
- Kiely M, Drum MA, Kessel W. Early discharge. Risks, benefits, and who decides. See comment in PubMed Commons below Clin Perinatol. 1998; 25: 539-553, vii-viii.
- Hebert PR, Reed G, Entman SS, Mitchel EF Jr, Berg C, Griffin MR, et al. Serious maternal morbidity after childbirth: prolonged hospital stays and readmissions. See comment in PubMed Commons below Obstet Gynecol. 1999; 94: 942-947.
- Howell EA, Mora P, Leventhal H. Correlates of early postpartum depressive symptoms. See comment in PubMed Commons below Matern Child Health J. 2006; 10: 149-157.
- Fortney JA, Smith JB. Measuring maternal morbidity. In: Safe Motherhood Initiatives: Critical Issues Reproductive Health Matters: Berer M, Ravindran TKS, editors. Blackwell Science. 1999.
- Leung KM, Elashoff RM, Rees KS, Hasan MM, Legorreta AP. Hospital- and patient-related characteristics determining maternity length of stay: a hierarchical linear model approach. See comment in PubMed Commons below Am J Public Health. 1998; 88: 377-381.